NDC Code(s) : 16714-115-01, 16714-115-02, 16714-116-01, 16714-116-02, 16714-117-01, 16714-117-02
Packager : Northstar Rx LLC.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| RivastigmineRivastigmine PATCH, EXTENDED RELEASE | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| RivastigmineRivastigmine PATCH, EXTENDED RELEASE | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| RivastigmineRivastigmine PATCH, EXTENDED RELEASE | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Northstar Rx LLC.(830546433) |
| REGISTRANT - Zydus Pharmaceuticals USA Inc.(156861945) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Zydus Lifesciences Limited | 650461283 | ANALYSIS(16714-115, 16714-116, 16714-117), MANUFACTURE(16714-115, 16714-116, 16714-117) | |
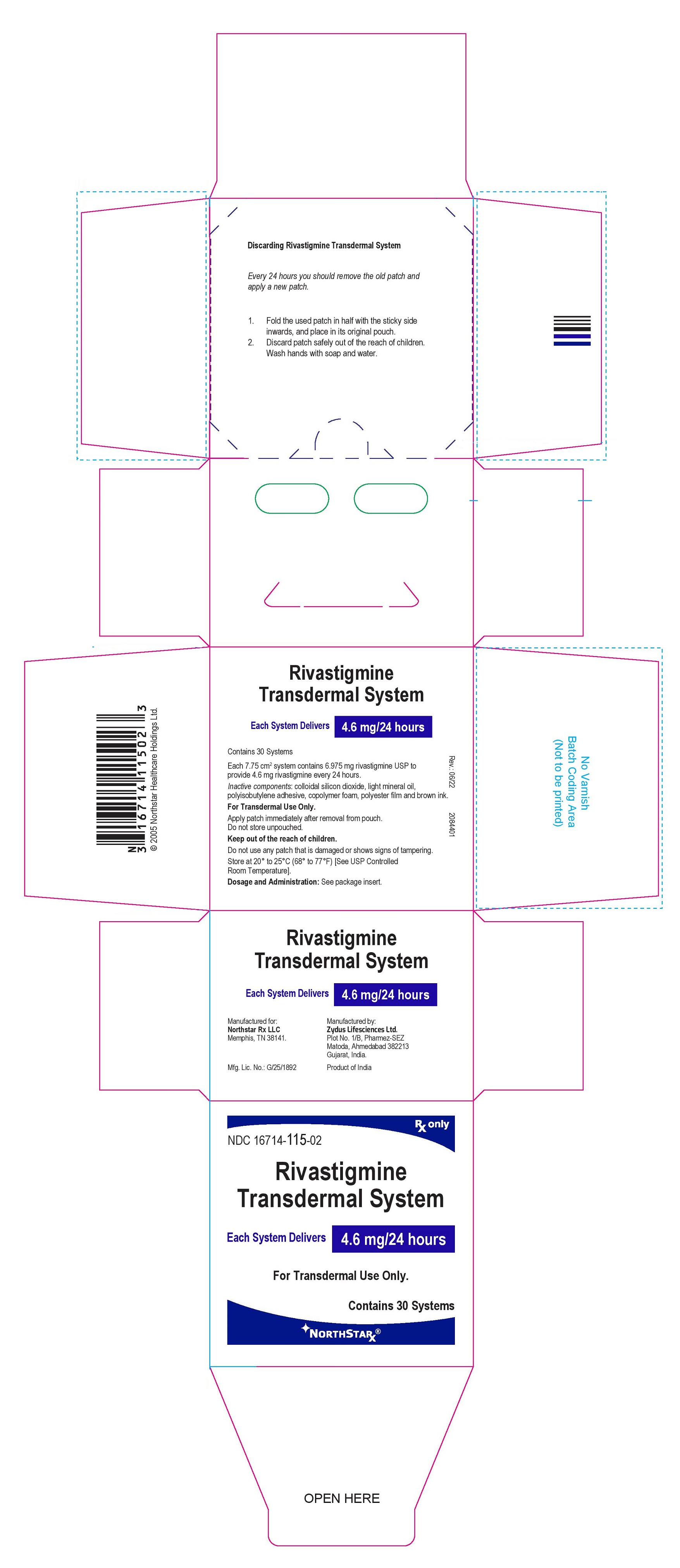
PRINCIPAL DISPLAY PANEL
Package Label – 4.6 mg / 24 hours
Rx only
NDC 16714-115-02
Rivastigmine
Transdermal System
Each System Delivers 4.6 mg/24 hours
For Transdermal Use Only.
Contains 30 systems
NORTHSTAR®
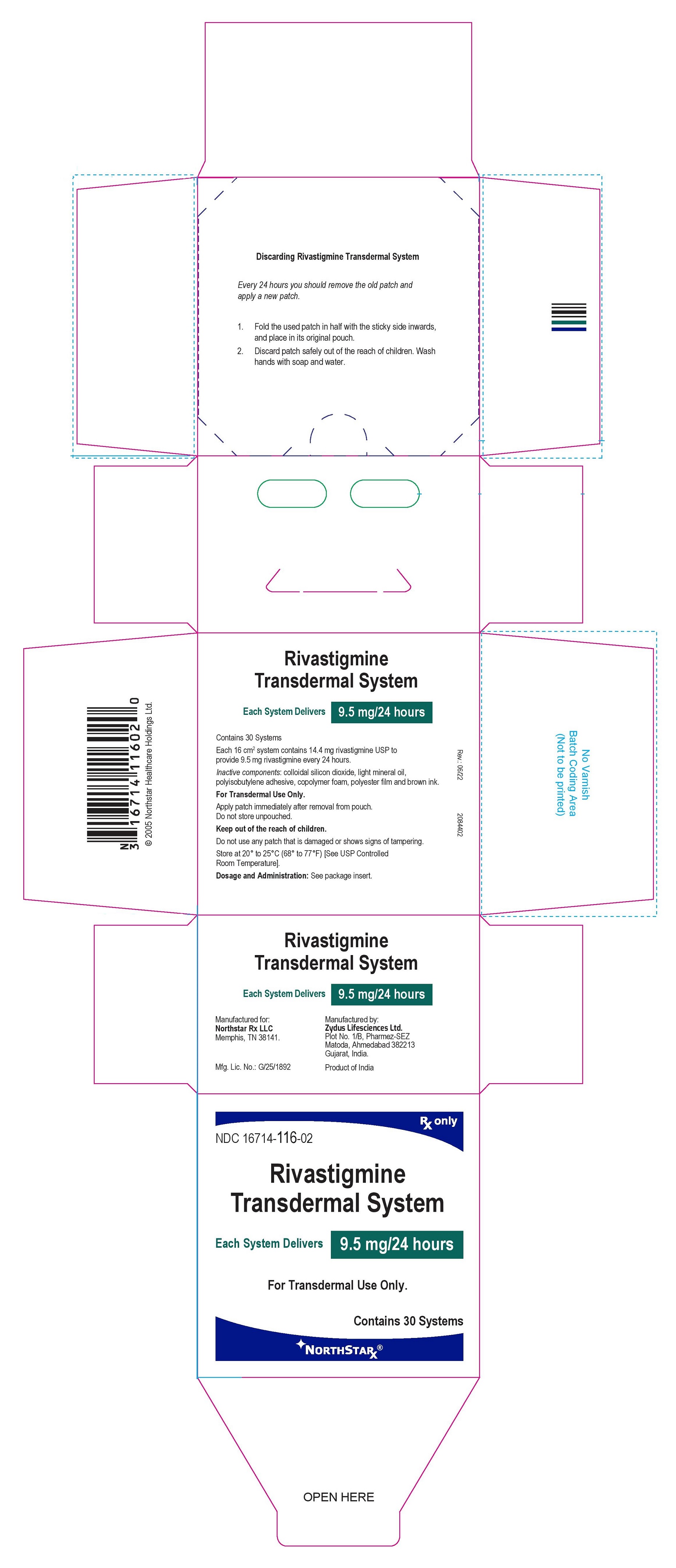
PRINCIPAL DISPLAY PANEL
Package Label – 9.5 mg / 24 hours
Rx only
NDC 16714-116-02
Rivastigmine
Transdermal System
Each System Delivers 9.5 mg/24 hours
For Transdermal Use Only.
Contains 30 systems
NORTHSTAR®
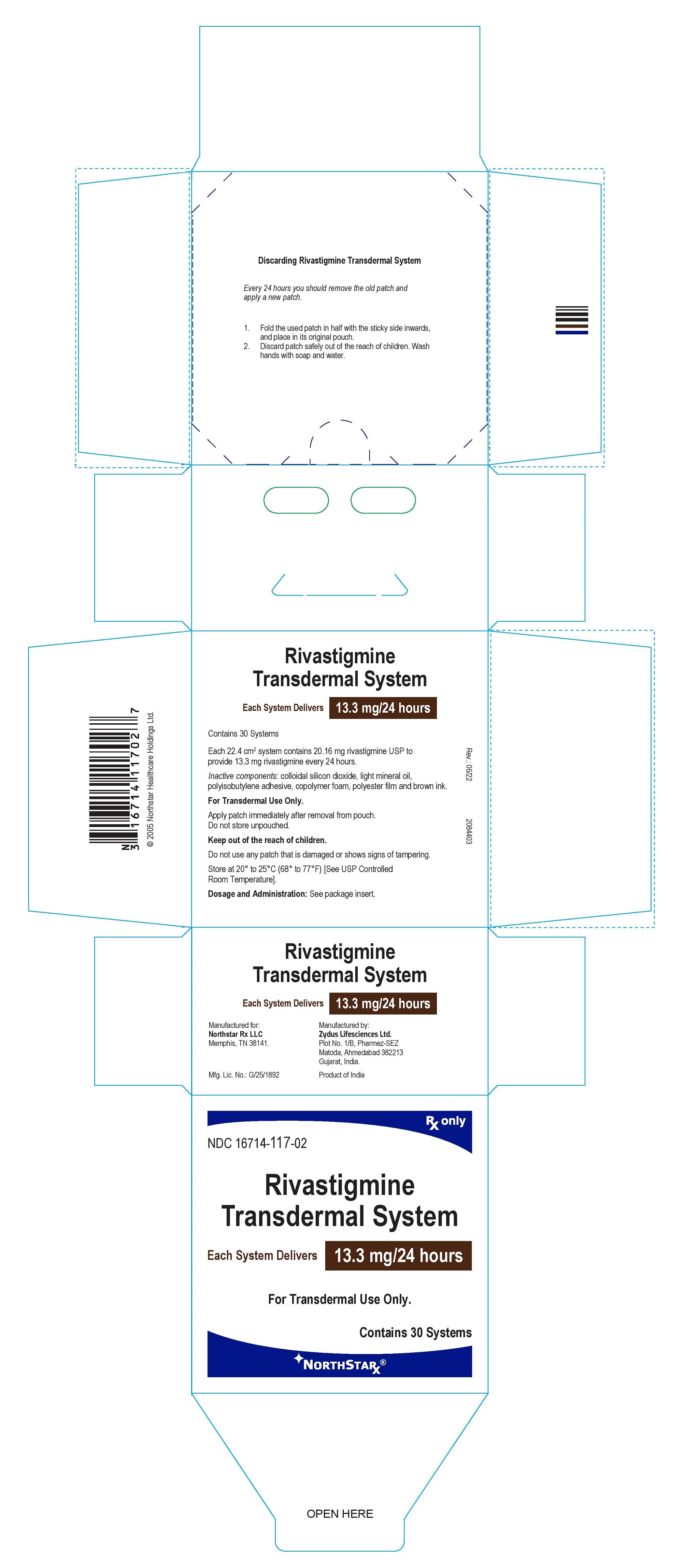
PRINCIPAL DISPLAY PANEL
Package Label – 13.3 mg / 24 hours
Rx only
NDC 16714-117-02
Rivastigmine
Transdermal System
Each System Delivers 13.3 mg/24 hours
For Transdermal Use Only.
Contains 30 systems
NORTHSTAR®