NDC Code(s) : 0517-6710-10
Packager : American Regent, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Calcium ChlorideCALCIUM CHLORIDE INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - American Regent, Inc.(002033710) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| American Regent, Inc. | 027223179 | manufacture(0517-6710) | |
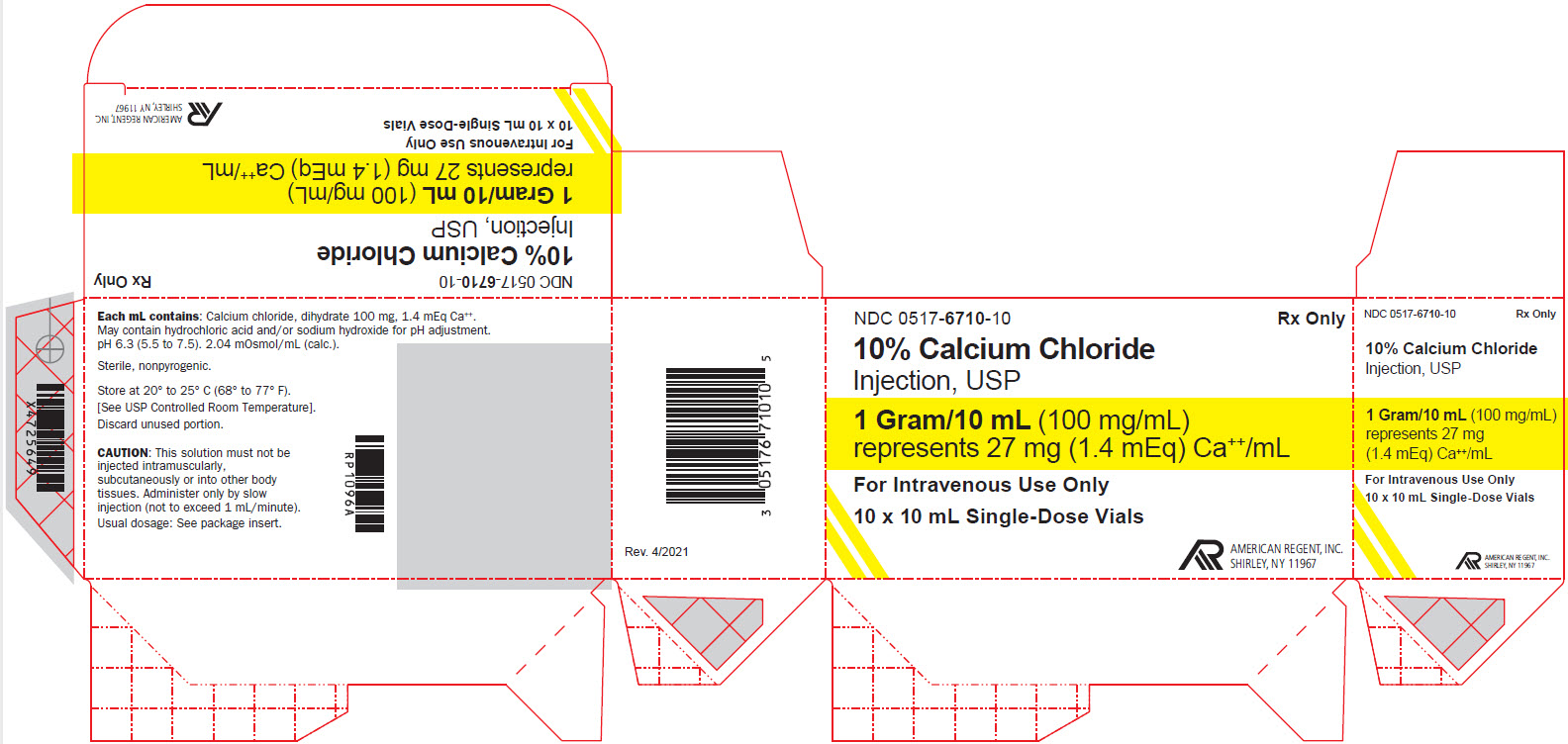
PRINCIPAL DISPLAY PANEL
NDC 0517-6710-01
Rx Only
10% Calcium Chloride Injection, USP
1 Gram/10 mL (100 mg/mL)
For Intravenous Use Only
Sterile, nonpyrogenic.
10 mL Single-dose Vial
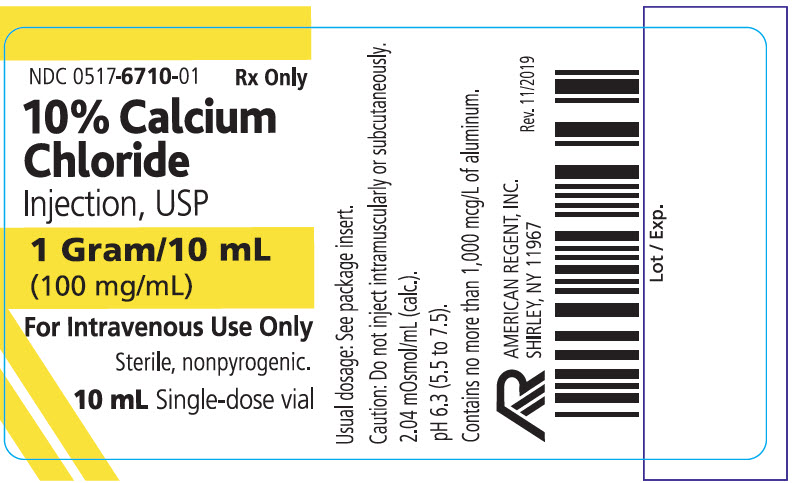
PRINCIPAL DISPLAY PANEL
NDC 0517-6710-10
Rx Only
10% Calcium Chloride Injection, USP
1 Gram/10 mL (100 mg/mL)
Represents 27 mg (1.4 mEq) Ca**/mL
For Intravenous Use Only
10 x 10 mL Single-dose Vials
American Regent, Inc.
Shirley, NY 11967