NDC Code(s) : 0517-4901-25, 0517-4905-25, 0517-4930-25
Packager : American Regent, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Dexamethasone Sodium PhosphateDexamethasone Sodium Phosphate INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Dexamethasone Sodium PhosphateDexamethasone Sodium Phosphate INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Dexamethasone Sodium PhosphateDexamethasone Sodium Phosphate INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
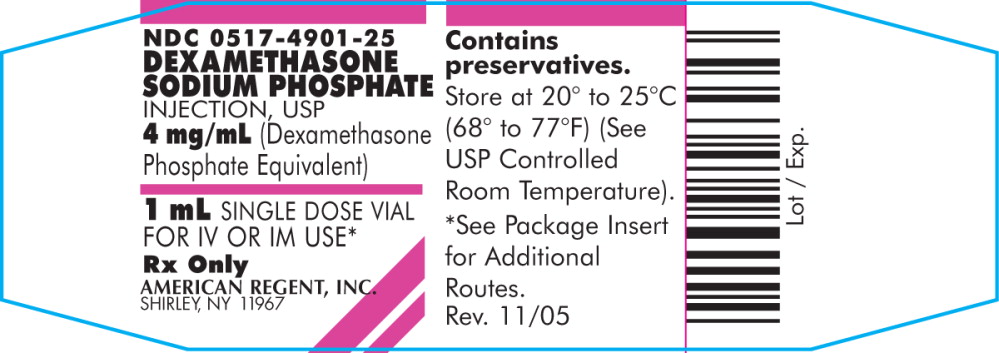
PRINCIPAL DISPLAY PANEL
NDC 0517-4901-25
DEXAMETHASONE
SODIUM PHOSPHATE
INJECTION, USP
4 mg/mL (Dexamethasone
Phosphate Equivalent)
1 mL SINGLE DOSE VIAL
FOR IV OR IM USE*
Rx Only
AMERICAN REGENT, INC.
SHIRLEY, NY 11967
PRINCIPAL DISPLAY PANEL
NDC 0517-4901-25
DEXAMETHASONE
SODIUM PHOSPHATE
INJECTION, USP
25 x 1 mL SINGLE DOSE VIALS
4 mg/mL (Dexamethasone Phosphate Equivalent)
Rx Only
FOR INTRAVENOUS, INTRAMUSCULAR, INTRA-ARTICULAR, SOFT TISSUE
OR INTRALESIONAL USE
Each mL contains: Dexamethasone Sodium Phosphate 4.37 mg (equivalent to
4 mg of Dexamethasone Phosphate), Sodium Sulfite 1 mg, Benzyl Alcohol
10 mg, Water for Injection q.s. Sodium Citrate for isotonicity. pH adjusted
with Citric Acid and/or Sodium Hydroxide.
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to
86°F) (See USP Controlled Room Temperature). Sensitive to heat - Do not autoclave.
Directions for Use: See Package Insert.
AMERICAN REGENT, INC.
SHIRLEY, NY 11967
Rev. 11/05

PRINCIPAL DISPLAY PANEL
NDC 0517-4905-25
DEXAMETHASONE
SODIUM PHOSPHATE
INJECTION, USP
20 mg/5 mL (4mg/mL)
(Dexamethasone Phosphate Equivalent)
5 mL MULTIPLE DOSE VIAL
FOR IV OR IM USE*
Rx Only
AMERICAN REGENT, INC.
SHIRLEY, NY 11967

PRINCIPAL DISPLAY PANEL
NDC 0517-4905-25
DEXAMETHASONE
SODIUM PHOSPHATE
INJECTION, USP
25 x 5 mL
MULTIPLE DOSE VIALS
20 mg/5 mL (4 mg/mL) (Dexamethasone Phosphate Equivalent)
Rx Only
FOR INTRAVENOUS, INTRAMUSCULAR, INTRA-ARTICULAR,
SOFT TISSUE OR INTRALESIONAL USE
Each mL contains: Dexamethasone Sodium Phosphate 4.37 mg (equivalent to 4 mg of
Dexamethasone Phosphate), Sodium Sulfite 1 mg, Benzyl Alcohol 10 mg, Water for Injection q.s.
Sodium Citrate for isotonicity. pH adjusted with Citric Acid and/or Sodium Hydroxide.
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) (See USP
Controlled Room Temperature). Sensitive to heat - Do not autoclave.
Directions for Use: See Package Insert.
AMERICAN REGENT, INC.
SHIRLEY, NY 11967
Rev. 5/09

PRINCIPAL DISPLAY PANEL
NDC 0517-4930-25
DEXAMETHASONE
SODIUM PHOSPHATE
INJECTION, USP
120 mg/30 mL (4 mg/mL)
(Dexamethasone Phosphate
Equivalent)
30 mL MULTIPLE DOSE VIAL
FOR IV, IM, INTRA-ARTICULAR,
SOFT TISSUE OR
INTRALESIONAL USE
Rx Only
AMERICAN REGENT, INC.
SHIRLEY, NY 11967









