NDC Code(s) : 0409-1920-10, 0409-1941-01
Packager : Hospira, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : CIV
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| TalwinPENTAZOCINE LACTATE INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| TalwinPENTAZOCINE LACTATE INJECTION, SOLUTION | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
10 mL Multi-Dose Vial
Talwin™
Injection
CIV
Pentazocine
Injection, USP
30 mg
base per mL
Printed in USA
Hospira, Inc., Lake Forest, IL 60045 USA

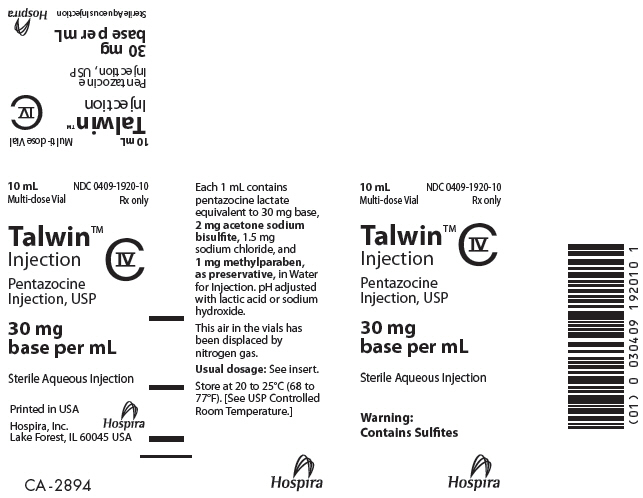
PRINCIPAL DISPLAY PANEL
10 mL
Multi-dose Vial
NDC 0409-1920-10
Rx only
Talwin™
Injection
CIV
Pentazocine
Injection, USP
30 mg
base per mL
Sterile Aqueous Injection
Warning:
Contains Sulfites
Hospira
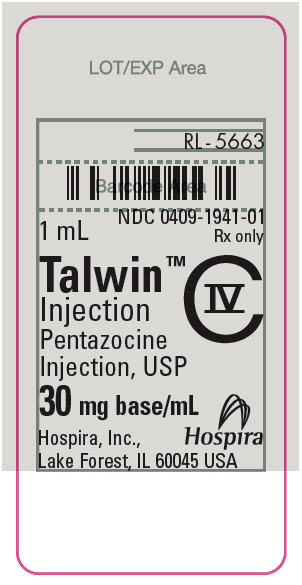
PRINCIPAL DISPLAY PANEL
1 mL
NDC 0409-1941-01
Rx only
Talwin™
Injection
Pentazocine
Injection, USP
CIV
30 mg base/mL
Hospira, Inc.,
Lake Forest, IL 60045 USA
Hospira
PRINCIPAL DISPLAY PANEL
1 mL UNI-AMP™ unit dose pak
Talwin™ Injection
Pentazocine Injection, USP
30 mg base/mL
Hospira
CIV
NDC 0409-1941-01
Hospira, Inc.,
Lake Forest, IL 60045 USA
Rx only
IM-2064

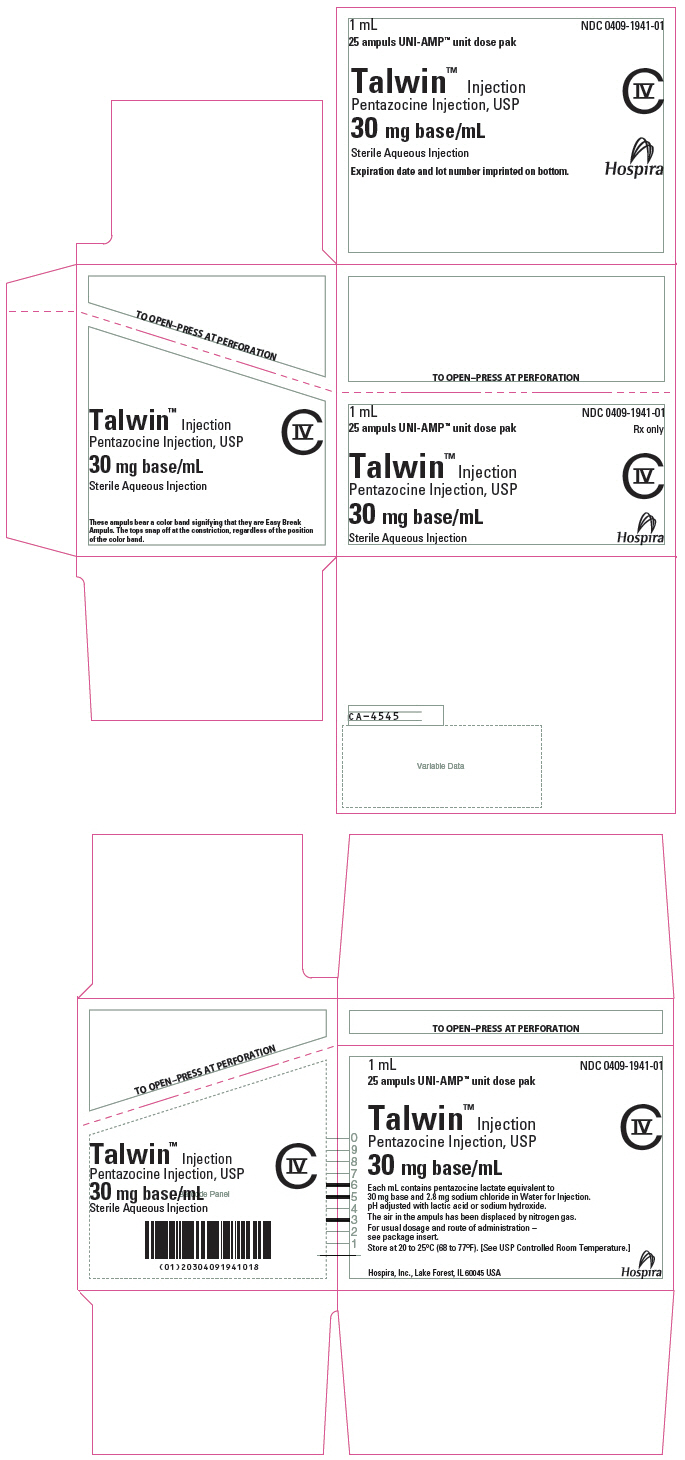
PRINCIPAL DISPLAY PANEL
1 mL
25 ampuls UNI-AMP™ unit dose pak
NDC 0409-1941-01
Rx only
Talwin™ Injection
Pentazocine Injection, USP
CIV
30 mg base/mL
Sterile Aqueous Injection
Hospira