NDC Code(s) : 0310-0401-60, 0310-0402-60
Packager : AstraZeneca Pharmaceuticals LP
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| ACCOLATEZafirlukast TABLET, FILM COATED | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| ACCOLATEZafirlukast TABLET, FILM COATED | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
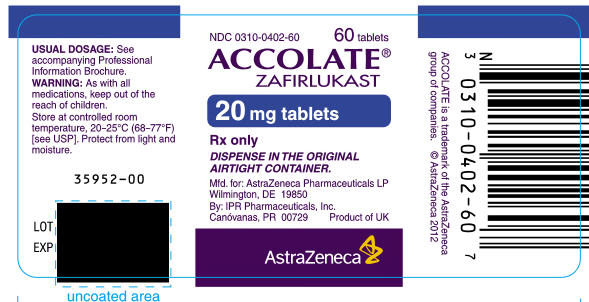
PRINCIPAL DISPLAY PANEL
NDC 0310-0401-60
60 tablets
ACCOLATE®
ZAFIRLUKAST
10 mg tablets
Rx only
DISPENSE IN THE ORIGINAL
AIRTIGHT CONTAINER.
Mfd. for: AstraZeneca Pharmaceuticals LP
Wilmington, DE 19850
By: IPR Pharmaceuticals, Inc.
Canóvanas, PR 00729
Product of UK
AstraZeneca
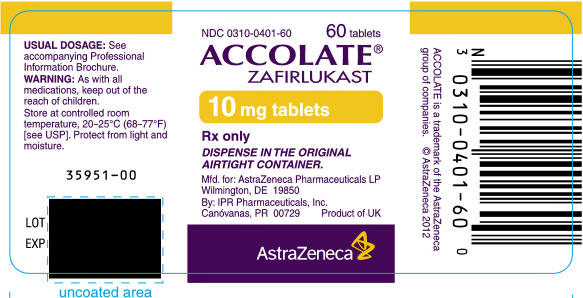
PRINCIPAL DISPLAY PANEL
NDC 0310-0402-60
60 tablets
ACCOLATE®
ZAFIRLUKAST
20 mg tablets
Rx only
DISPENSE IN THE ORIGINAL
AIRTIGHT CONTAINER.
Mfd. for: AstraZeneca Pharmaceuticals LP
Wilmington, DE 19850
By: IPR Pharmaceuticals, Inc.
Canóvanas, PR 00729
Product of UK
AstraZeneca