NDC Code(s) : 0245-0035-89, 0245-0035-30, 0245-0035-01, 0245-0037-89, 0245-0037-30, 0245-0037-01
Packager : Upsher-Smith Laboratories, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Klor-ConPotassium Chloride POWDER, FOR SOLUTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Klor-ConPotassium Chloride POWDER, FOR SOLUTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
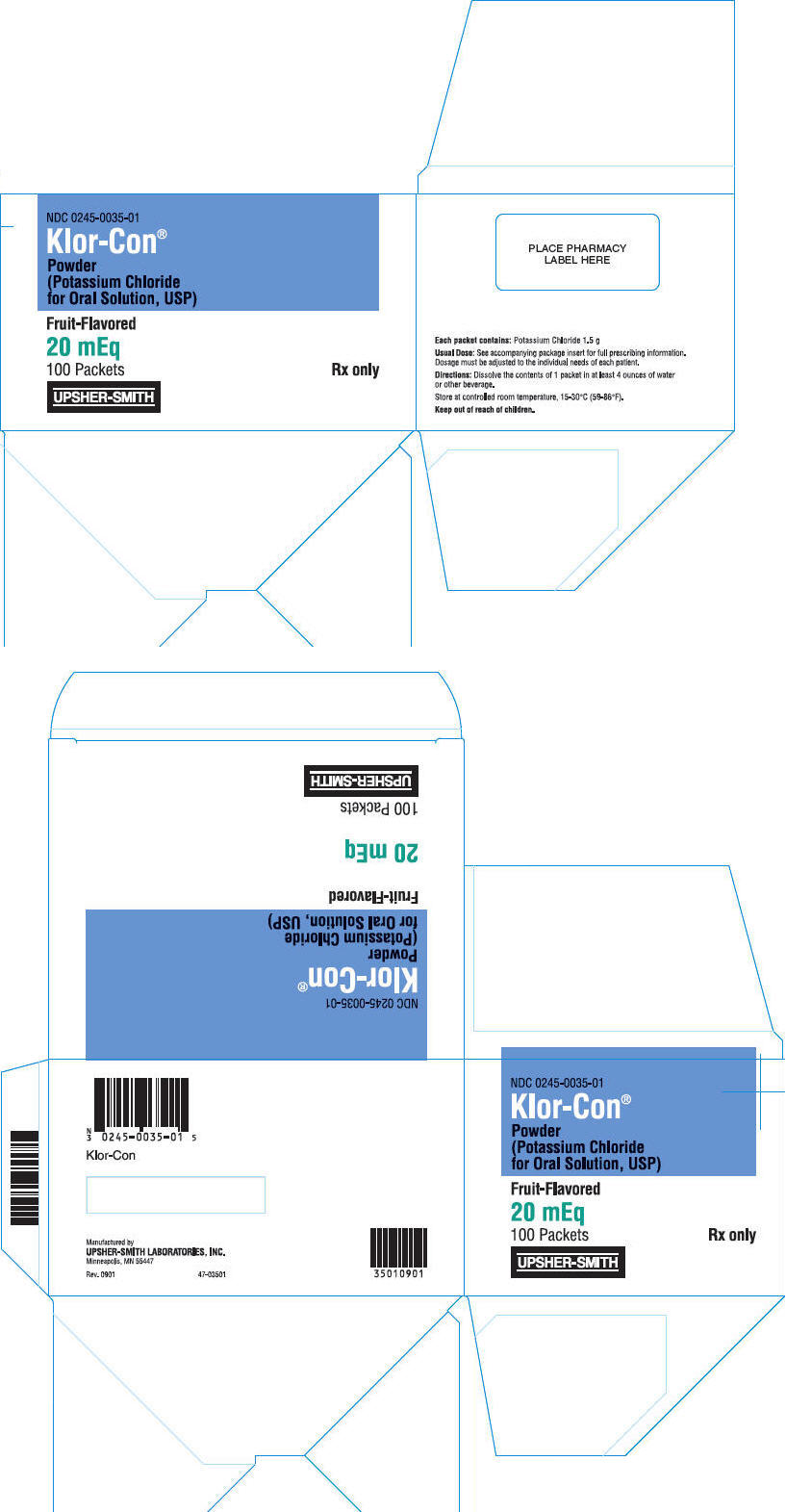
PRINCIPAL DISPLAY PANEL
NDC 0245-0035-01
Klor-Con®
Powder
(Potassium Chloride
for Oral Solution, USP)
Fruit-Flavored
20 mEq
100 Packets
Rx only
UPSHER-SMITH
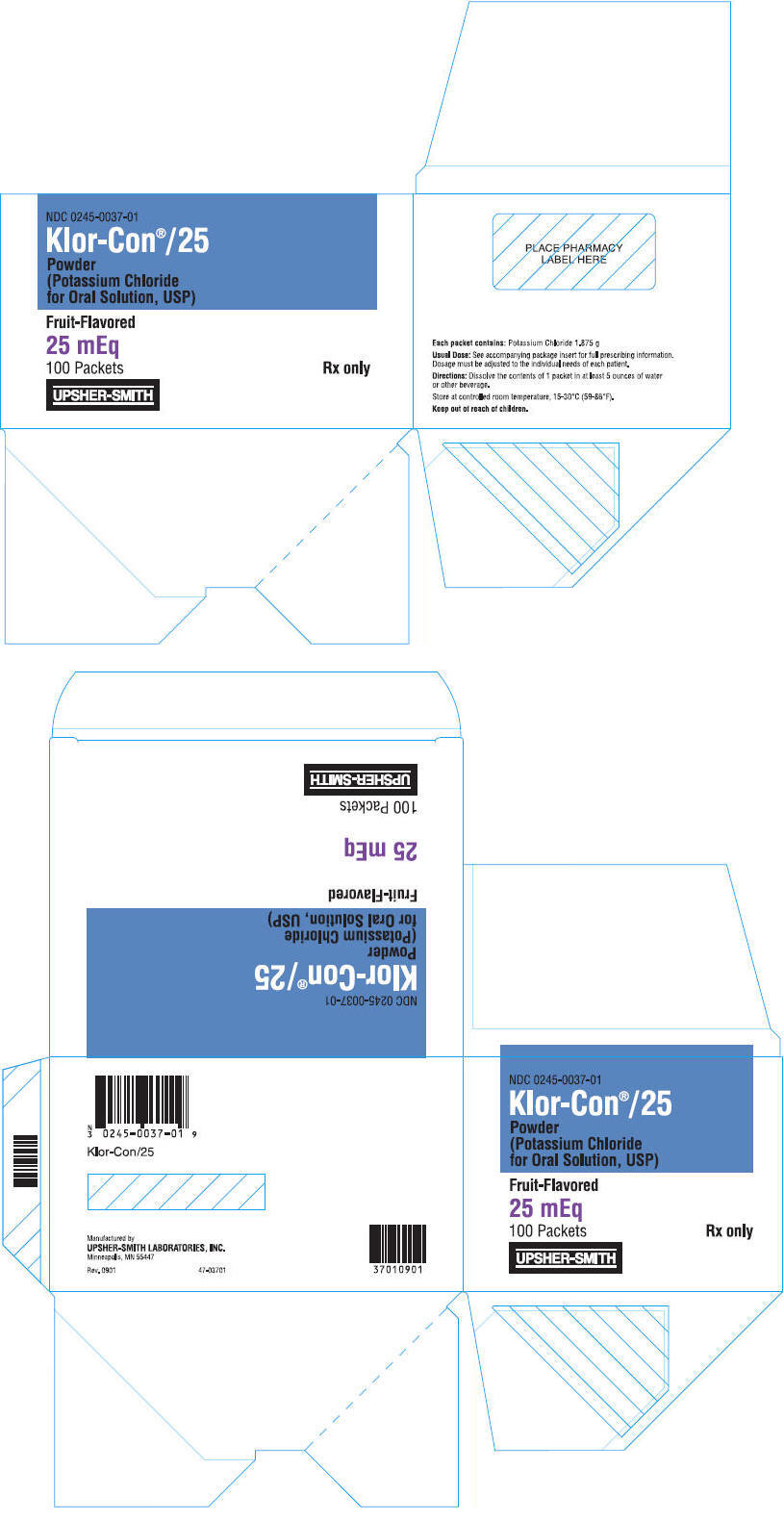
PRINCIPAL DISPLAY PANEL
NDC 0245-0037-01
Klor-Con®/25
Powder
(Potassium Chloride
for Oral Solution, USP)
Fruit-Flavored
25 mEq
100 Packets
Rx only
UPSHER-SMITH