NDC Code(s) : 0228-3156-03, 0228-3153-03
Packager : Actavis Pharma, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : CIII
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| BuprenorphineBuprenorphine TABLET | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| BuprenorphineBuprenorphine TABLET | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| LABELER - Actavis Pharma, Inc.(119723554) |
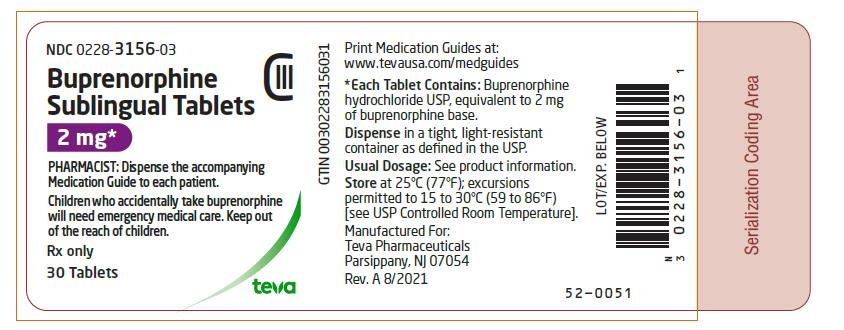
PRINCIPAL DISPLAY PANEL
NDC 0228-3156-03
Buprenorphine Sublingual Tablets 2 mg* CIII
PHARMACIST: Dispense the accompanying Medication Guide to each patient.
Children who accidentally take buprenorphine will need emergency medical care. Keep out of the reach of children.
Rx only
30 Tablets
PRINCIPAL DISPLAY PANEL
NDC 0228-3153-03
Buprenorphine Sublingual Tablets 8 mg* CIII
PHARMACIST: Dispense the accompanying Medication Guide to each patient.
Children who accidentally take buprenorphine will need emergency medical care. Keep out of the reach of children.
Rx only
30 Tablets









