NDC Code(s) : 0186-0777-08, 0186-0777-60, 0186-0777-18, 0186-0777-39, 0186-0777-28, 0186-0776-60, 0186-0776-94
Packager : AstraZeneca Pharmaceuticals LP
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| BRILINTATicagrelor TABLET | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| BRILINTATicagrelor TABLET | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| LABELER - AstraZeneca Pharmaceuticals LP(938368834) |
| REGISTRANT - AstraZeneca PLC(230790719) |
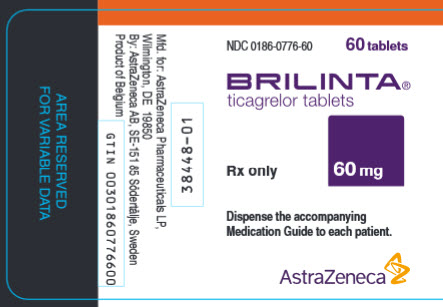
PRINCIPAL DISPLAY PANEL
NDC 0186-0776-60
60 tablets
BRILINTA®
ticagrelor tablets
60 mg
Rx only
Dispense the accompanying Medication Guide to each patient.
AstraZeneca
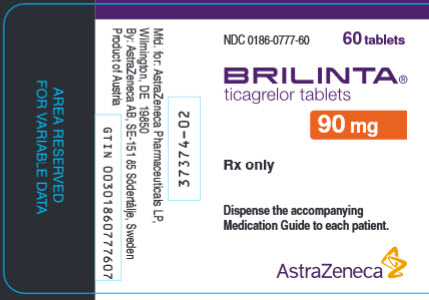
PRINCIPAL DISPLAY PANEL
NDC 0186-0777-60
60 tablets
BRILINTA®
ticagrelor tablets
90 mg
Rx only
Dispense the accompanying Medication Guide to each patient.
AstraZeneca