NDC Code(s) : 0169-7704-21, 0169-7704-92, 0169-7705-21, 0169-7705-92, 0169-7708-21, 0169-7708-92, 0169-7703-11, 0169-7703-91, 0169-7703-21, 0169-7703-92
Packager : Novo Nordisk
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Norditropinsomatropin INJECTION, SOLUTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Norditropinsomatropin INJECTION, SOLUTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Norditropinsomatropin INJECTION, SOLUTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Norditropinsomatropin INJECTION, SOLUTION | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| LABELER - Novo Nordisk(622920320) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Novo Nordisk A/S - Hax | 306711800 | MANUFACTURE(0169-7704, 0169-7705, 0169-7708, 0169-7703), API MANUFACTURE(0169-7704, 0169-7705, 0169-7708, 0169-7703) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Novo Nordisk A/S - Kv | 311359009 | MANUFACTURE(0169-7704, 0169-7705, 0169-7708, 0169-7703) | |
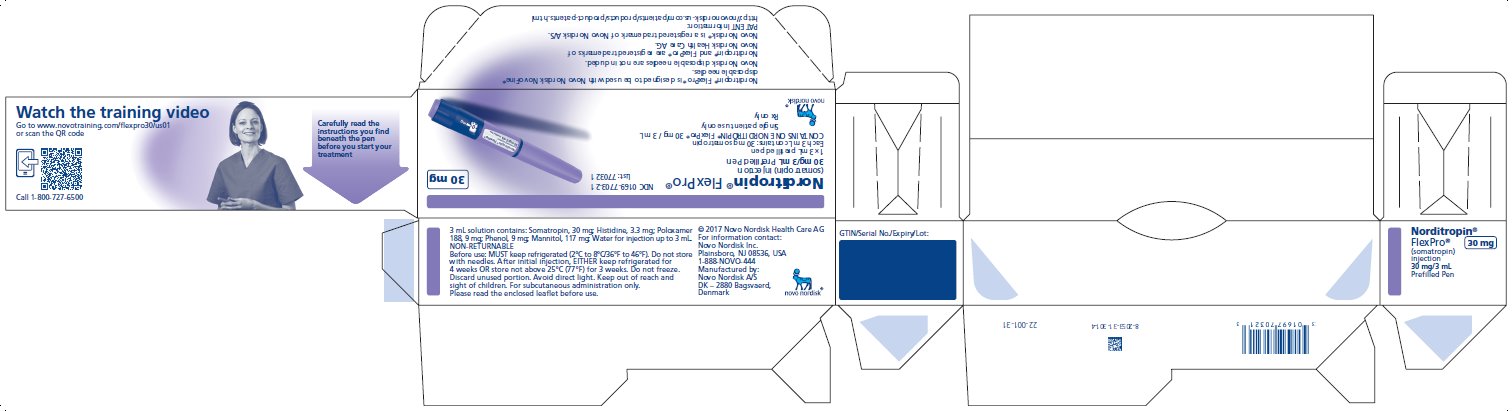
PRINCIPAL DISPLAY PANEL
Norditropin® FlexPro®
(somatropin) injection
5 mg/1.5 mL Prefilled Pen
1 x 1.5 mL prefilled pen
Each 1.5 mL contains 5 mg somatropin
CONTAINS ONE
NORDITROPIN® FlexPro® 5 mg/1.5 mL
Single patient use only
Rx only
NDC 0169-7704-21
List: 770421
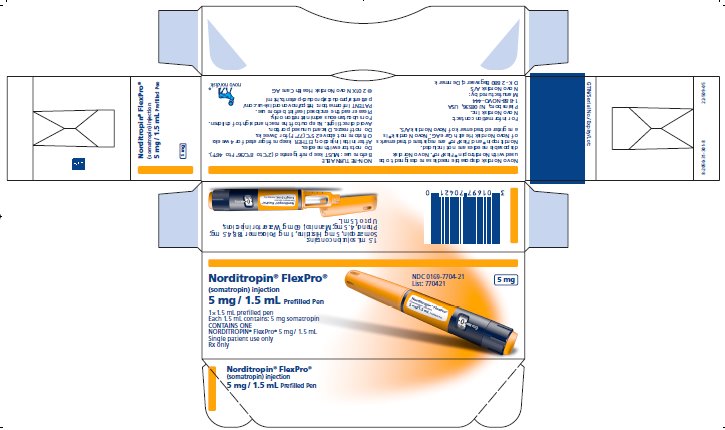
PRINCIPAL DISPLAY PANEL
Norditropin® FlexPro®
(somatropin) injection
10 mg/1.5 mL Prefilled Pen
1 x 1.5 mL prefilled disposable pen
Each 1.5 mL contains 10 mg somatropin
CONTAINS ONE
NORDITROPIN® FlexPro® 10 mg/1.5 mL
Single patient use only
Rx only
NDC 0169-7705-21
List: 770521
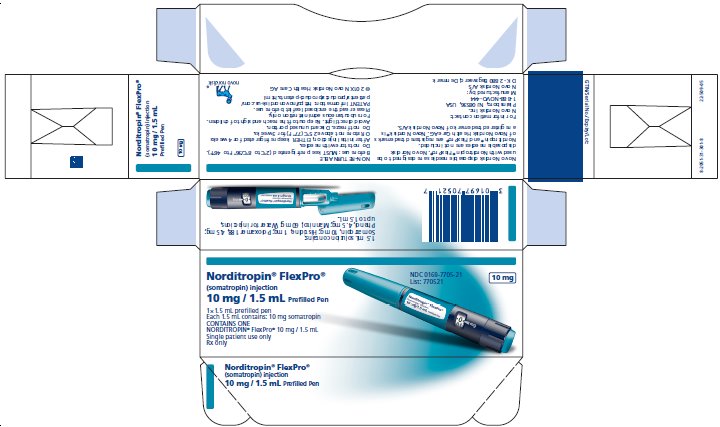
PRINCIPAL DISPLAY PANEL
Norditropin® FlexPro®
(somatropin) injection
15 mg/1.5 mL Prefilled Pen
1 x 1.5 mL prefilled disposable pen
Each 1.5 mL contains 15 mg somatropin
CONTAINS ONE
NORDITROPIN® FlexPro® 15 mg/1.5 mL
Single patient use only
Rx only
NDC 0169-7708-21
List: 770821
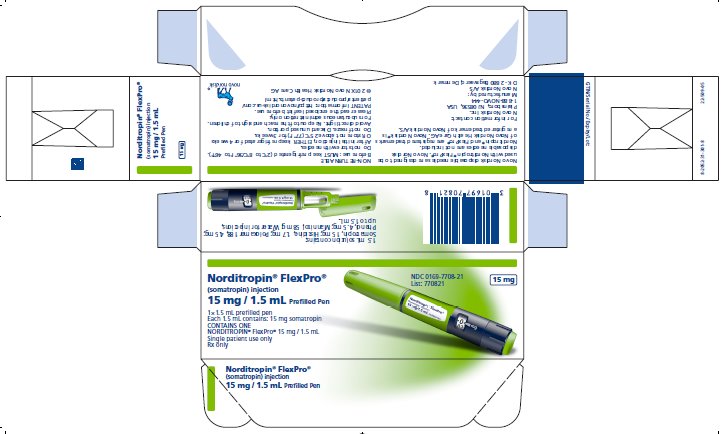
PRINCIPAL DISPLAY PANEL
Norditropin® FlexPro®
(somatropin) injection
30 mg / 3 mLPrefilled Pen
1x3 mL prefilled pen
Each 3 mL contains: 30 mg somatropin
CONTAINS ONE NORDITROPIN® FlexPro® 30 mg / 3 mL
Single patient use only
Rx only
NDC 0169-7703-21
List: 770321