NDC Code(s) : 0169-3685-12, 0169-3685-92, 0169-3696-19, 0169-3696-97, 0169-3696-90, 0169-3696-98, 0169-2200-11, 0169-2201-25
Packager : Novo Nordisk
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| NovoLog Mix 70/30insulin aspart INJECTION, SUSPENSION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| NovoLog Mix 70/30insulin aspart INJECTION, SUSPENSION | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| NovoLog Mix 70/30insulin aspart INJECTION, SUSPENSION | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| NovoLog Mix 70/30insulin aspart INJECTION, SUSPENSION | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| LABELER - Novo Nordisk(622920320) |
PRINCIPAL DISPLAY PANEL
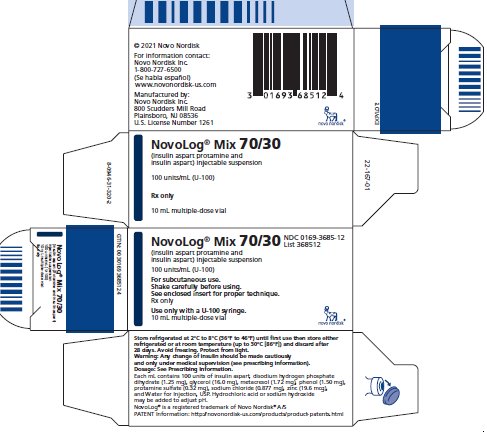
NDC 0169-3685-12
List 368512
NovoLog® Mix 70/30
(insulin aspart protamine and
insulin aspart) injectable suspension
100 units/mL (U-100)
For subcutaneous use.
Shake carefully before using.
See enclosed insert for proper technique.
Rx only
Use only with a U-100 syringe.
10 mL multiple-dose vial
PRINCIPAL DISPLAY PANEL
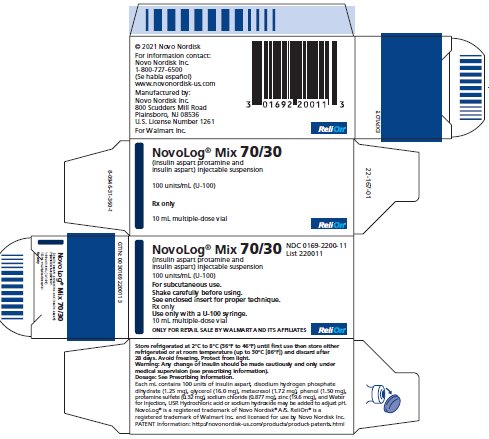
NDC 0169-2200-11
List 220011
NovoLog® Mix 70/30
(insulin aspart protamine
and insulin aspart) injectable suspension
100 units/mL (U-100)
For subcutaneous use.
Shake carefully before using.
See enclosed insert for proper technique.
Rx only
Use only with a U-100 syringe.
10 mL multiple-dose vial
ONLY FOR RETAIL SALE BY WALMART
AND ITS AFFILIATES
ReliOn ®
PRINCIPAL DISPLAY PANEL
NDC 0169-3696-19
List 369619
NovoLog® Mix 70/30
FlexPen® Prefilled Pen
(insulin aspart protamine and
insulin aspart) injectable suspension
For Single Patient Use Only
100 units/mL (U-100)
5×3 mL prefilled pens
For subcutaneous use.
Shake carefully before using.
See enclosed insert for proper technique.
For use with NovoFine®, NovoFine® Plus or NovoTwist® disposable needles.
Store refrigerated at 2°C to 8°C (36°F to 46°F) until first use. After first use store at room temperature (up to 30°C [86°F]) and discard after 14 days. Avoid freezing. Protect from light.
Rx only
Dispense in this sealed carton.

PRINCIPAL DISPLAY PANEL
NDC 0169-2201-25
List 220125
NovoLog® Mix 70/30
FlexPen® Prefilled Pen
(insulin aspart protamine and
insulin aspart) injectable suspension
For Single Patient Use Only
100 units/mL (U-100)
5×3 mL prefilled pens
For subcutaneous use.
Shake carefully before using.
See enclosed insert for proper technique.
For use with NovoFine®, NovoFine® Plus or NovoTwist® disposable needles.
Store refrigerated at 2°C to 8°C (36°F to 46°F) until first use.
After first use store at room temperature (up to 30°C [86°F]) and discard after 14 days. Avoid freezing. Protect from light.
Rx only
Dispense in this sealed carton.
ONLY FOR RETAIL SALE BY WALMART AND ITS AFFILIATES
ReliOn ®









