NDC Code(s) : 0143-9886-01, 0143-9886-50, 0143-9886-75, 0143-9887-01, 0143-9887-50, 0143-9887-75
Packager : Hikma Pharmaceuticals USA Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| AmoxicillinAmoxicillin POWDER, FOR SUSPENSION | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| AmoxicillinAmoxicillin POWDER, FOR SUSPENSION | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| LABELER - Hikma Pharmaceuticals USA Inc.(001230762) |
PRINCIPAL DISPLAY PANEL
Principal Display Panel
NDC 0143-9886-01
Amoxicillin
For Oral Suspension, USP
Dye Free
200 mg/5 mL*
100 mL (when reconstituted)
WARNING: NOT FOR INJECTION
Rx Only

PRINCIPAL DISPLAY PANEL
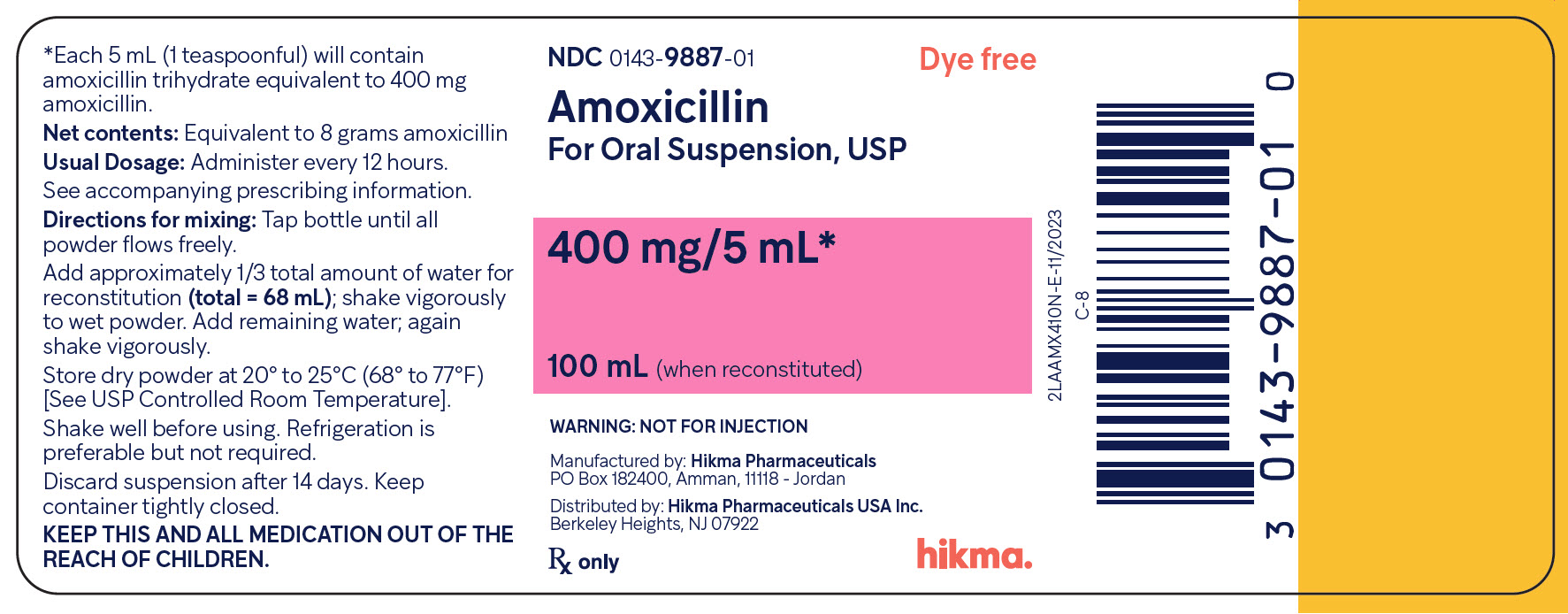
Principal Display Panel
NDC 0143-9887-01
Amoxicillin
For Oral Suspension, USP
Dye Free
400 mg/5 mL*
100 mL (when reconstituted)
WARNING: NOT FOR INJECTION
Rx Only