NDC Code(s) : 0085-0571-02, 0085-1110-01, 0085-0539-01
Packager : Merck Sharp & Dohme Corp.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| INTRON AInterferon alfa-2b KIT | |||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| INTRON AInterferon alfa-2b KIT | |||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| INTRON AInterferon alfa-2b KIT | |||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
PRINCIPAL DISPLAY PANEL
SCHERING
NDC 0085-0571-02
Refrigerate
Interferon alfa-2b,
recombinant
Intron
® A
Powder for Injection
Single-Use Vial
10 million IU per vial
For Intramuscular/Subcutaneous/Intravenous/
Intralesional Use.
Rx only
Medication Guide for patient enclosed.
Dosage and Administration:
See package insert.
Reconstitute as directed in package insert.
Read accompanying directions.

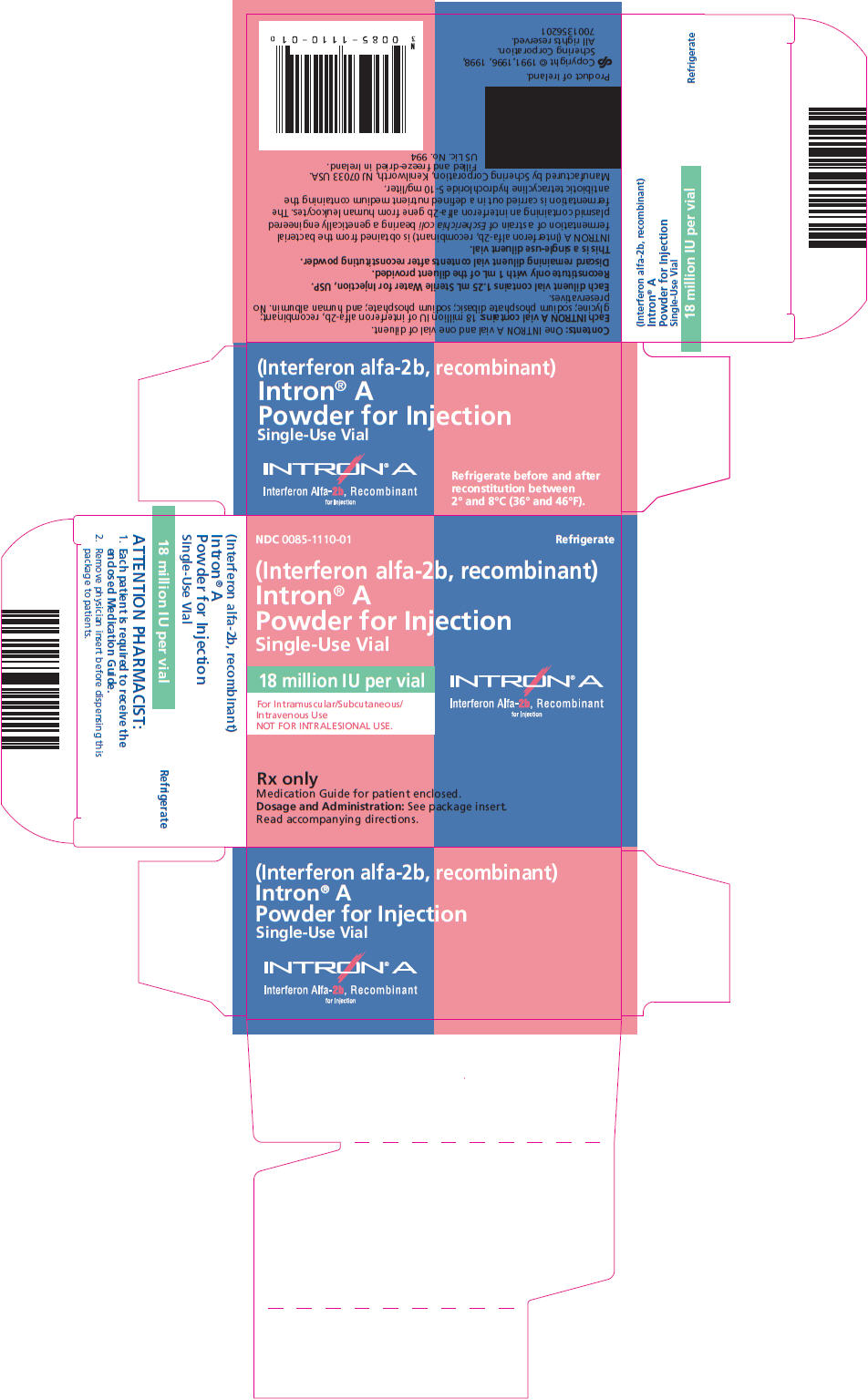
PRINCIPAL DISPLAY PANEL
NDC 0085-1110-01
Refrigerate
(Interferon alfa-2b, recombinant)
Intron® A
Powder for Injection
Single-Use Vial
18 million IU per vial
For Intramuscular/Subcutaneous/
Intravenous Use
NOT FOR INTRALESIONAL USE.
INTRON® A
Interferon Alfa-2b, Recombinant
for Injection
Rx only
Medication Guide for patient enclosed.
Dosage and Administration: See package insert.
Read accompanying directions.
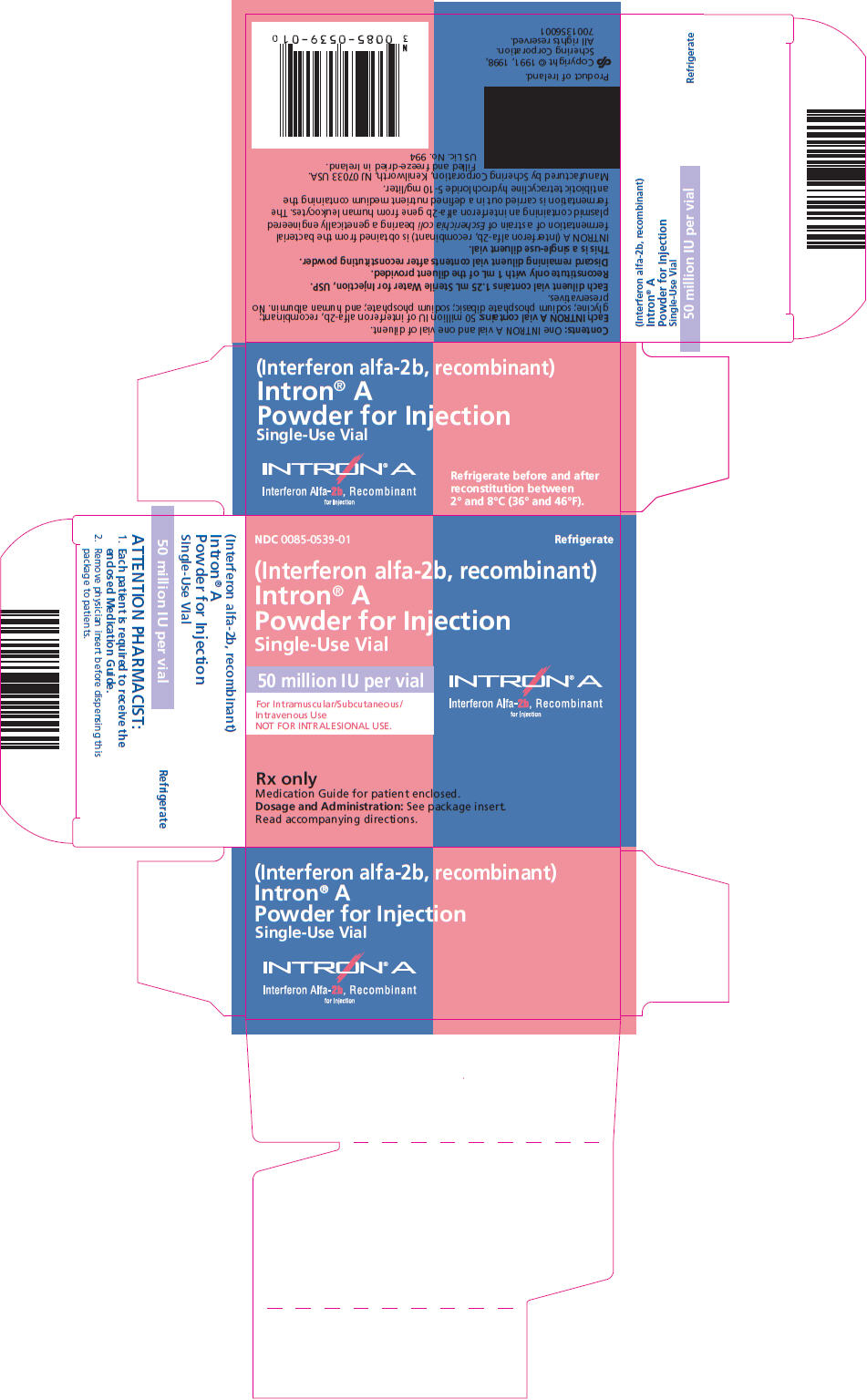
PRINCIPAL DISPLAY PANEL
NDC 0085-0539-01
Refrigerate
(Interferon alfa-2b, recombinant)
Intron® A
Powder for Injection
Single-Use Vial
50 million IU per vial
For Intramuscular/Subcutaneous/
Intravenous Use
NOT FOR INTRALESIONAL USE.
INTRON® A
Interferon Alfa-2b, Recombinant
for Injection
Rx only
Medication Guide for patient enclosed.
Dosage and Administration: See package insert.
Read accompanying directions.