NDC Code(s) : 0069-0006-02, 0069-0006-01, 0069-0006-04, 0069-0006-03
Packager : Pfizer Laboratories Div Pfizer Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| EtomidateEtomidate INJECTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
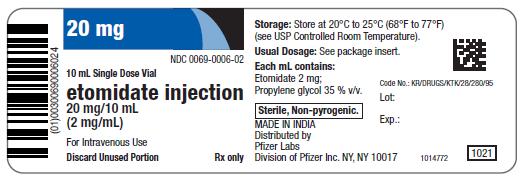
20 mg
NDC 0069-0006-02
10 mL Single Dose Vial
etomidate injection
20 mg/10 mL
(2 mg/mL)
For Intravenous Use
Discard Unused Portion
Rx only
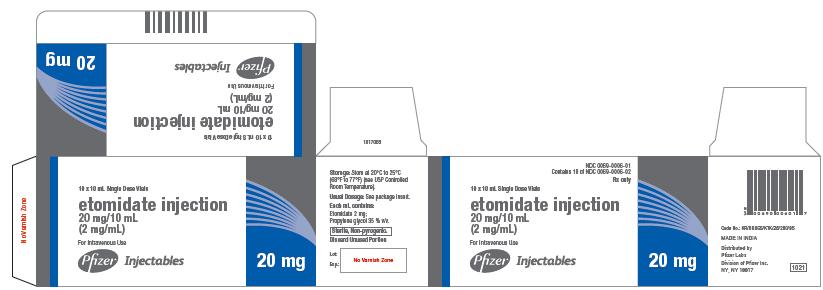
NDC 0069-0006-01
Contains 10 of NDC 0069-0006-02
Rx only
10 x 10 mL Single Dose Vials
etomidate injection
20 mg/10 mL
(2 mg/mL)
For Intravenous Use
Pfizer Injectables
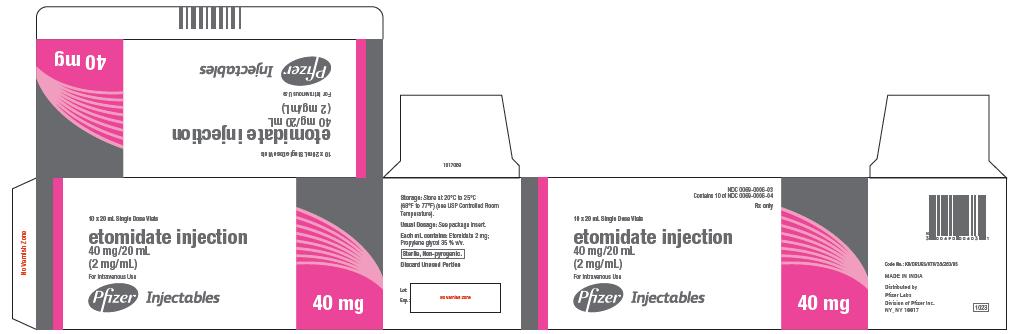
40 mg
NDC 0069-0006-04
20 mL Single Dose Vial
etomidate injection
40 mg/20 mL
(2 mg/mL)
For Intravenous Use
Discard Unused Portion
Rx only
Pfizer Injectables
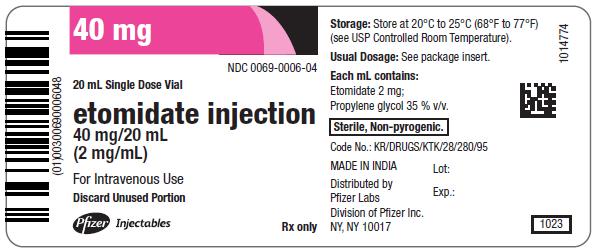
NDC 0069-0006-03
Contains 10 of NDC 0069-0006-04
Rx only
10 x 20 mL Single Dose Vials
etomidate injection
40 mg/20 mL
(2 mg/mL)
For Intravenous Use
Pfizer Injectables