NDC Code(s) : 0002-7715-01, 0002-7715-59, 0002-7715-63, 0002-8214-01, 0002-8214-05
Packager : Eli Lilly and Company
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| BASAGLAR Insulin glargine INJECTION, SOLUTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| BASAGLAR Insulin glargine INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Eli Lilly and Company(006421325) |
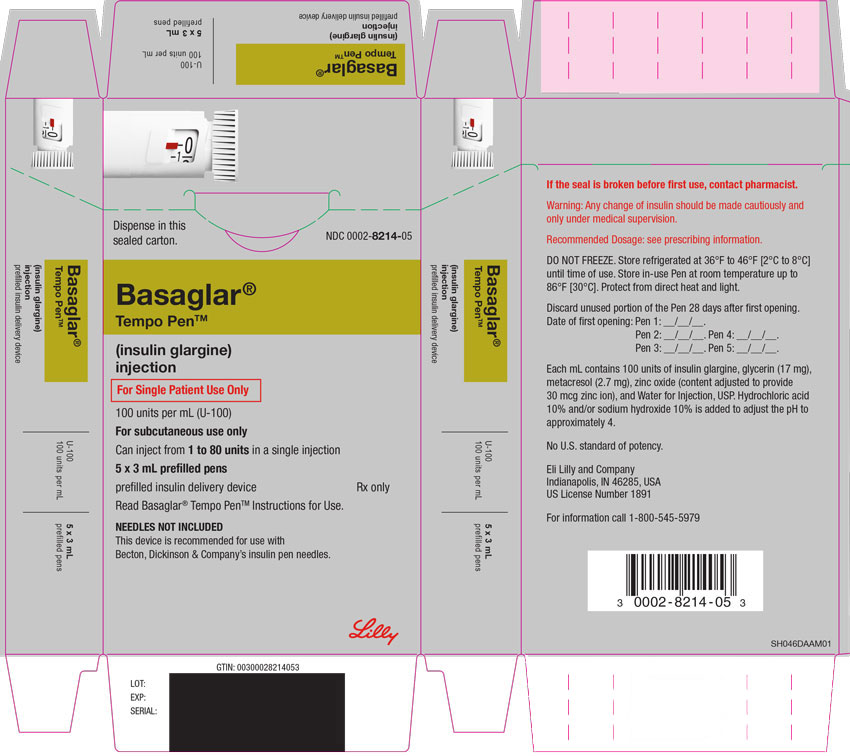
PRINCIPAL DISPLAY PANEL
PACKAGE CARTON – BASAGLAR KwikPen 80 UNIT PEN
Dispense in this sealed carton.
NDC 0002-7715-59
Basaglar®
KwikPen®
(insulin glargine)
injection
For Single Patient Use Only
100 units per mL (U-100)
For subcutaneous use only
Can inject from 1 to 80 units in a single injection
5 x 3 mL prefilled pens
prefilled insulin delivery device
Rx only
Read Basaglar® KwikPen® Instructions for Use.
NEEDLES NOT INCLUDED
This device is recommended for use with Becton, Dickinson & Company's insulin pen needles.
Lilly
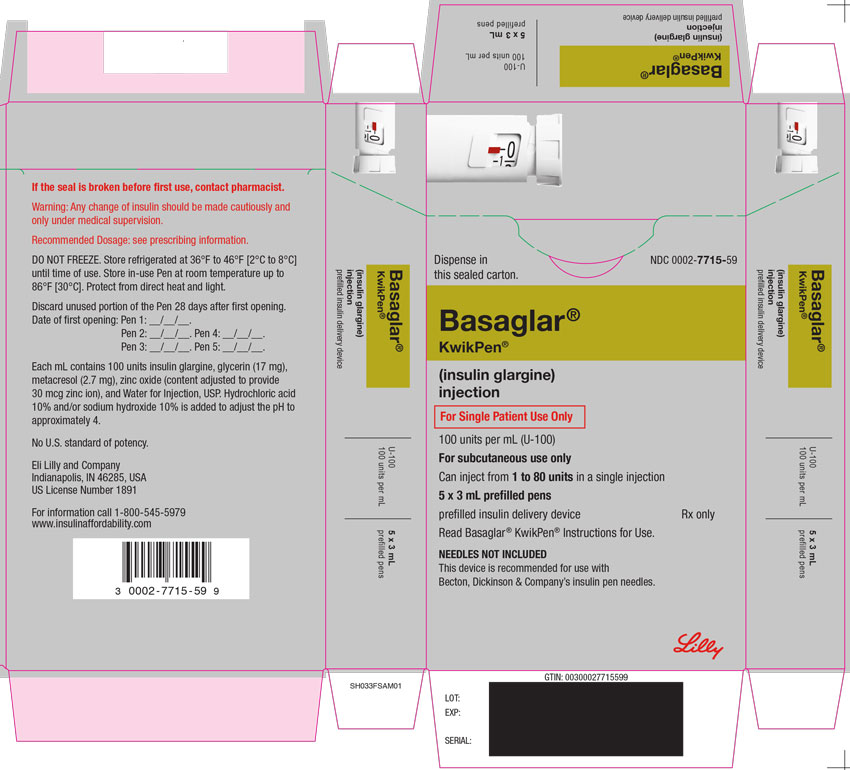
PRINCIPAL DISPLAY PANEL
PACKAGE CARTON – BASAGLAR Tempo Pen 80 UNIT PEN
Dispense in this sealed carton.
NDC 0002-8214-05
Basaglar®
Tempo Pen™
(insulin glargine)
injection
For Single Patient Use Only
100 units per mL (U-100)
For subcutaneous use only
Can inject from 1 to 80 units in a single injection
5 x 3 mL prefilled pens
prefilled insulin delivery device
Rx only
Read Basaglar® Tempo Pen™ Instructions for Use.
NEEDLES NOT INCLUDED
This device is recommended for use with Becton, Dickinson & Company's insulin pen needles.
Lilly