
Synopsis
Synopsis
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
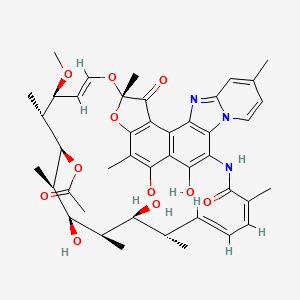

1. 4-deoxy-4'-methylpyrido(1',2'-1,2)imidazo(5,4c)rifamycin
2. L 105
3. L-105
4. L105
5. Redactiv
6. Xifaxan
1. Rifaxidin
2. Rifacol
3. 80621-81-4
4. Rifamycin L 105
5. Xifaxan
6. Rifamycin L 105sv
7. Fatroximin
8. Rifaximine
9. Normix
10. Rifaximina
11. Xifaxsan
12. L-105
13. Rifamixin
14. Rifaximine [french]
15. Rifaximinum [latin]
16. Rifaximina [spanish]
17. Ritacol
18. Chebi:75246
19. 4-deoxy-4'-methylpyrido(1',2'-1,2)imidazo(5,4-c)rifamycin Sv
20. L36o5t016n
21. Rifaximin (xifaxan)
22. Nsc-758957
23. Rifaximinum
24. Brn 3584528
25. (2s,16z,18e,20s,21s,22r,23r,24r,25s,26r,27s,28e)-5,6,21,23-tetrahydroxy-27-methoxy-2,4,11,16,20,22,24,26-octamethyl-1,15-dioxo-1,2-dihydro-2,7-(epoxypentadeca[1,11,13]trienoimino)furo[2'',3'':7',8']naphtho[1',2':4,5]imidazo[1,2-a]pyridin-25-yl Acetate
26. C43h51n3o11
27. Rifaxin
28. Redactiv
29. Ido[1,2-a]benzimidazol-25-yl Acetate
30. (2s,16z,18e,20s,21s,22r,23r,24r,25s,26r,27s,28e)-25-(acetyloxy)-5,6,21,23-tetrahydroxy-27-methoxy-2,4,11,16,20,22,24,26-octamethyl-2,7-(epoxypentadeca[1,11,13]trienimino)benzofuro[4,5-e]pyrido[1,2-a]benzimidazole-1,15(2h)-dione
31. L 105sv
32. L 105 (ansamacrolide Antibiotic)
33. L 105
34. Rifaximinun
35. Flonorm
36. Lumenax
37. Spiraxin
38. Lormyx
39. Rifaximin [usan:inn:ban]
40. Unii-l36o5t016n
41. 5-yl Acetate
42. Ncgc00095842-01
43. (2s,16z,18e,20s,21s,22r,23r,24r,25s,26r,27s,28e)-25-(acetyloxy)-5,6,21,23-tetrahydroxy-27-methoxy-2,4,11,16,20,22,24,26-octamethyl-2,7-(epoxypentadeca(1,11,13)trienimino)benzofuro(4,5-e)pyrido(1,2-a)benzimidazole-1,15(2h)-dione
44. (2s,16z,18e,20s,21s,22r,23r,24r,25s,26r,27s,28e)5,6,21,23-tetrahydroxy-27-methoxy-2,4,11,16,20,22,24,26-octamethyl-1,15-dioxo-1,2-dihydro-2,7-(epoxypentadeca[1,11,13]trienoimino)[1]benzofuro[4,5-e]pyr
45. Xifaxan (tn)
46. Mfcd00864973
47. Rifaximin [inn]
48. Rifaximin [jan]
49. Rifaximin [mi]
50. Rifaximin [usan]
51. Rifaximin [vandf]
52. Rifaximin [mart.]
53. Alpha-0817185
54. Rifaximin [who-dd]
55. Chembl1617
56. Dsstox_cid_25998
57. Dsstox_rid_81280
58. Dsstox_gsid_45998
59. (2s,16z,18e,20s,21s,22r,23r,24r,25s,26s,27s,28e)-5,6,21,23,25 Pentahydroxy-27-methoxy-2,4,11,16,20,22,24,26-octamethyl-2,7-(epoxypentadeca(1,11,13)trienimino)benzofuro(4,5-e)pyrido(1,2-a)benzimidazole-1,15(2h)-dione, 25-acetate
60. Rifaximin (jan/usan/inn)
61. Schembl124066
62. Rifaximin [ep Impurity]
63. Rifaximin [orange Book]
64. Dtxsid7045998
65. Rifaximin [ep Monograph]
66. Gtpl12012
67. Hms3715b19
68. 88747-56-2
69. Tox21_111529
70. Bdbm50347620
71. S1790
72. Akos015963053
73. Zinc169621200
74. Ccg-221129
75. Db01220
76. Nsc 758957
77. Rifaximin 100 Microg/ml In Acetonitrile
78. 2,7-(epoxy(1,11,13)pentadecatrienoimino)furo(2'',3'':7',8')naphth(1',2':4,5)imidazo(1,2-a)pyridine-1,15(2h)-dione, 25-(acetyloxy)-5,6,21,23-tetrahydroxy-27-methoxy-2,4,11,16,20,22,24,26-octamethyl-, ( 2s,16z,18e,20s,21s,22r,23r,24r,25s,26r,27s,28e)-
79. 2,7-(epoxypentadeca(1,11,13)trienimino)benzofuro(4,5-e)pyrido(1,2-a)benzimidazole-1,15(2h)-dione, 25-(acetyloxy)-5,6,21,23-tetrahydroxy-27-methoxy-2,4,11,16,20,22,24,26-octamethyl-, (2s-(2r*,16z,18e,20r*,21r*,22s*,23s*,24s*,25r*,26s*,27r*,28e))-
80. Ac-19112
81. Cas-80621-81-4
82. L/105
83. D02554
84. Ab01209738-01
85. Ab01209738-03
86. Ab01209738_04
87. Rifaximin, Antibiotic For Culture Media Use Only
88. 621r814
89. Q416073
90. Q-201671
91. (2s,16z,18e,20s,21s,22r,23r,24r,25s,26r,27s,28e)-5,6,21,23-tetrahydroxy-27-methoxy-2,4,11,16,20,22,24,26-octamethyl-1,1
92. (7s,11s,12r,13s,14r,15r,16r,17s,18s)-2,15,17,36-tetrahydroxy-11-methoxy-3,7,12,14,16,18,22,30-octamethyl-6,23-dioxo-8,37-dioxa-24,27,33-triazahexacyclo[23.10.1.1^{4,7}.0^{5,35}.0^{26,34}.0^{27,32}]heptatriaconta-1,3,5(35),9,19,21,25(36),26(34),28,30,32-undecaen-13-yl Acetate
93. [(7s,9e,11s,12r,13s,14r,15r,16r,17s,18s,19e,21z)-2,15,17,36-tetrahydroxy-11-methoxy-3,7,12,14,16,18,22,30-octamethyl-6,23-dioxo-8,37-dioxa-24,27,33-triazahexacyclo[23.10.1.14,7.05,35.026,34.027,32]heptatriaconta-1(35),2,4,9,19,21,25(36),26(34),28,30,32-undecaen-13-yl] Acetate
94. 2,7-(epoxypentadeca(1,11,13)trienimino)benzofuro(4,5-e)pyrido(1,2-a)benzimidazole-1,15(2h)-dione, 25-(acetyloxy)-5,6,21,23-tetrahydroxy-27-methoxy-2,4,11,16,20,22,24,26-octamethyl-, (2s,16z,18e,20s,21s,22r,23r,24r,25s,26r,27s,28e)-
95. 5-dioxo-1,2-dihydro-2,7-(epoxypentadeca[1,11,13]trienoimino)furo[2'',3'':7',8']naphtho[1',2':4,5]imidazo[1,2-a]pyridin-2
| Molecular Weight | 785.9 g/mol |
|---|---|
| Molecular Formula | C43H51N3O11 |
| XLogP3 | 6.9 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 3 |
| Exact Mass | 785.35235945 g/mol |
| Monoisotopic Mass | 785.35235945 g/mol |
| Topological Polar Surface Area | 198 Ų |
| Heavy Atom Count | 57 |
| Formal Charge | 0 |
| Complexity | 1590 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 3 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Xifaxan |
| PubMed Health | Rifaximin (By mouth) |
| Drug Classes | Antibiotic |
| Drug Label | XIFAXAN tablets contain rifaximin, a non-aminoglycoside semi-synthetic, nonsystemic antibiotic derived from rifamycin SV. Rifaximin is a structural analog of rifampin. The chemical name for rifaximin is (2 ,16 ,18 ,20 ,21 ,22 ,23 ,24 ,25 ,26 ,27... |
| Active Ingredient | Rifaximin |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; 550mg |
| Market Status | Prescription |
| Company | Salix Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Xifaxan |
| PubMed Health | Rifaximin (By mouth) |
| Drug Classes | Antibiotic |
| Drug Label | XIFAXAN tablets contain rifaximin, a non-aminoglycoside semi-synthetic, nonsystemic antibiotic derived from rifamycin SV. Rifaximin is a structural analog of rifampin. The chemical name for rifaximin is (2 ,16 ,18 ,20 ,21 ,22 ,23 ,24 ,25 ,26 ,27... |
| Active Ingredient | Rifaximin |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; 550mg |
| Market Status | Prescription |
| Company | Salix Pharms |
Rifaximin has multiple indications by the FDA: for the treatment of patients (12 years of age) with traveller's diarrhea caused by noninvasive strains of Escherichia coli; for the reduction of overt hepatic encephalopathy recurrence in patients 18 years of age; and in May 2015 it was approved for irritable bowel syndrome with diarrhea (IBS-D) treatment in adult men and women.
FDA Label
Rifaximin is a structural analog of rifampin and a non-systemic, gastrointestinal site-specific antibiotic. This non-systemic property of the drug is due to the addition of a pyridoimidazole ring, which renders it non-absorbable. Rifaximin acts by inhibiting bacterial ribonucleic acid (RNA) synthesis and contributes to restore intestinal microflora imbalance. Other studies have also shown rifaximin to be an pregnane X receptor (PXR) activator. As PXR is responsible for inhibiting the proinflammatory transcription factor NF-kappa B (NF-B) and is inhibited in inflammatory bowel disease (IBD), rifaximin was proven to be effective for the treatment of IBS-D.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Gastrointestinal Agents
Drugs used for their effects on the gastrointestinal system, as to control gastric acidity, regulate gastrointestinal motility and water flow, and improve digestion. (See all compounds classified as Gastrointestinal Agents.)
A07AA11
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A07 - Antidiarrheals, intestinal antiinflammatory/antiinfective agents
A07A - Intestinal antiinfectives
A07AA - Antibiotics
A07AA11 - Rifaximin
D - Dermatologicals
D06 - Antibiotics and chemotherapeutics for dermatological use
D06A - Antibiotics for topical use
D06AX - Other antibiotics for topical use
D06AX11 - Rifaximin
Absorption
Low absorption in both the fasting state and when administered within 30 minutes of a high-fat breakfast.
Route of Elimination
In a mass balance study, after administration of 400 mg 14C-rifaximin orally to healthy volunteers, of the 96.94% total recovery, 96.62% of the administered radioactivity was recovered in feces almost exclusively as the unchanged drug and 0.32% was recovered in urine mostly as metabolites with 0.03% as the unchanged drug.Rifaximin accounted for 18% of radioactivity in plasma. This suggests that the absorbed rifaximin undergoes metabolism with minimal renal excretion of the unchanged drug
In vitro drug interactions studies have shown that rifaximin, at concentrations ranging from 2 to 200 ng/mL, did not inhibit human hepatic cytochrome P450 isoenzymes: 1A2, 2A6, 2B6, 2C9, 2C19, 2D6, 2E1, and 3A4. In an in vitro hepa-tocyte induction model, rifaximin was shown to induce cytochrome P450 3A4 (CYP3A4), an isoenzyme which rifampin is known to induce.
Approximately 6 hours.
Rifaximin acts by inhibiting RNA synthesis in susceptible bacteria by binding to the beta-subunit of bacterial deoxyribonucleic acid (DNA)-dependent ribonucleic acid (RNA) polymerase enzyme. This binding blocks translocation, which stops transcription.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Rifaximin is a antibiotic drug candidate, which is currently being evaluated in Phase IV clinical studies for the treatment of Diarrhea.
Lead Product(s): Rifaximin,Oral Rehydration Therapy (ORT)
Therapeutic Area: Gastroenterology Brand Name: Undisclosed
Study Phase: Phase IVProduct Type: Antibiotic
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 16, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Rifaximin,Oral Rehydration Therapy (ORT)
Therapeutic Area : Gastroenterology
Highest Development Status : Phase IV
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Rifaximin 200 mg Plus Oral Rehydration vs Oral Rehydration Alone in Children With Acute Diarrhea
Details : Rifaximin is a antibiotic drug candidate, which is currently being evaluated in Phase IV clinical studies for the treatment of Diarrhea.
Product Name : Undisclosed
Product Type : Antibiotic
Upfront Cash : Inapplicable
December 16, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Xifaxan-Generic (Rifaximin) is a Antibiotic drug candidate, which is currently being evaluated in Approved FDF clinical studies for the treatment of Hepatic Encephalopathy.
Lead Product(s): Rifaximin,Inapplicable
Therapeutic Area: Neurology Brand Name: Xifaxan-Generic
Study Phase: Approved FDFProduct Type: Antibiotic
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable October 07, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Rifaximin,Inapplicable
Therapeutic Area : Neurology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Biocon unit gets USFDA nod for genetic anti-bacterial drug
Details : Xifaxan-Generic (Rifaximin) is a Antibiotic drug candidate, which is currently being evaluated in Approved FDF clinical studies for the treatment of Hepatic Encephalopathy.
Product Name : Xifaxan-Generic
Product Type : Antibiotic
Upfront Cash : Inapplicable
October 07, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Xifaxan-Generic (rifaximin) is a rifamycin antibacterial indicated for the treatment of irritable bowel syndrome with diarrhoea (IBS-D) in adults.
Lead Product(s): Rifaximin,Inapplicable
Therapeutic Area: Gastroenterology Brand Name: Xifaxan-Generic
Study Phase: Approved FDFProduct Type: Antibiotic
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable June 02, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Rifaximin,Inapplicable
Therapeutic Area : Gastroenterology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Zydus gets USFDA nod for generic IBS-D treatment drug
Details : Xifaxan-Generic (rifaximin) is a rifamycin antibacterial indicated for the treatment of irritable bowel syndrome with diarrhoea (IBS-D) in adults.
Product Name : Xifaxan-Generic
Product Type : Antibiotic
Upfront Cash : Inapplicable
June 02, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Rifaximin is a Antibiotic drug candidate, which is currently being evaluated in phase I/ phase II clinical studies for the treatment of Alzheimer Disease.
Lead Product(s): Rifaximin,Inapplicable
Therapeutic Area: Neurology Brand Name: Undisclosed
Study Phase: Phase I/ Phase IIProduct Type: Antibiotic
Sponsor: Jasmohan Bajaj
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 05, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Rifaximin,Inapplicable
Therapeutic Area : Neurology
Highest Development Status : Phase I/ Phase II
Partner/Sponsor/Collaborator : Jasmohan Bajaj
Deal Size : Inapplicable
Deal Type : Inapplicable
Rifaximin SSD in Dementia Trial
Details : Rifaximin is a Antibiotic drug candidate, which is currently being evaluated in phase I/ phase II clinical studies for the treatment of Alzheimer Disease.
Product Name : Undisclosed
Product Type : Antibiotic
Upfront Cash : Inapplicable
December 05, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Vedolizumab is a Antibody drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Pouchitis.
Lead Product(s): Vedolizumab,Ciprofloxacin,Metronidazole,Vancomycin Hydrochloride,Amoxicillin Clavulanate,Rifaximin
Therapeutic Area: Trauma (Emergency, Injury, Surgery) Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Antibody, Unconjugated
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable June 05, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Vedolizumab,Ciprofloxacin,Metronidazole,Vancomycin Hydrochloride,Amoxicillin Clavulanate,Rifaximin
Therapeutic Area : Trauma (Emergency, Injury, Surgery)
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Vedolizumab is a Antibody drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Pouchitis.
Product Name : Undisclosed
Product Type : Antibody, Unconjugated
Upfront Cash : Inapplicable
June 05, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Rifaximin is a Antibiotic drug candidate, which is currently being evaluated in phase IV clinical studies for the treatment of Pouchitis.
Lead Product(s): Rifaximin,Inapplicable
Therapeutic Area: Trauma (Emergency, Injury, Surgery) Brand Name: Undisclosed
Study Phase: Phase IVProduct Type: Antibiotic
Sponsor: Bausch Health
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable March 15, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Rifaximin,Inapplicable
Therapeutic Area : Trauma (Emergency, Injury, Surgery)
Highest Development Status : Phase IV
Partner/Sponsor/Collaborator : Bausch Health
Deal Size : Inapplicable
Deal Type : Inapplicable
Rifaximin for the Secondary Prevention of Recurrent Pouchitis
Details : Rifaximin is a Antibiotic drug candidate, which is currently being evaluated in phase IV clinical studies for the treatment of Pouchitis.
Product Name : Undisclosed
Product Type : Antibiotic
Upfront Cash : Inapplicable
March 15, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
XIFAXAN (rifaximin) 200 mg tablets are indicated for travelers diarrhea. XIFAXAN (rifaximin) 550 mg tablets are indicated for reduction in risk of overt hepatic encephalopathy recurrence in adults and for the treatment of irritable bowel syndrome with diarrhea in adults.
Lead Product(s): Rifaximin,Inapplicable
Therapeutic Area: Gastroenterology Brand Name: Xifaxan-Generic
Study Phase: Approved FDFProduct Type: Antibiotic
Sponsor: Bausch Health
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable September 09, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Rifaximin,Inapplicable
Therapeutic Area : Gastroenterology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Bausch Health
Deal Size : Inapplicable
Deal Type : Inapplicable
Bausch Health Responds to Norwich Pharmaceuticals Tentative FDA Approval for a 200 mg Rifaximin
Details : XIFAXAN (rifaximin) 200 mg tablets are indicated for travelers diarrhea. XIFAXAN (rifaximin) 550 mg tablets are indicated for reduction in risk of overt hepatic encephalopathy recurrence in adults and for the treatment of irritable bowel syndrome with di...
Product Name : Xifaxan-Generic
Product Type : Antibiotic
Upfront Cash : Inapplicable
September 09, 2022

Details:
Rifaximin is a Antibiotic drug candidate, which is currently being evaluated in phase II clinical studies for the treatment of Lung Neoplasms.
Lead Product(s): Rifaximin,Fecal Microbiota
Therapeutic Area: Oncology Brand Name: Undisclosed
Study Phase: Phase IIProduct Type: Antibiotic
Sponsor: Biotax Labs | Israel Cancer Association
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable August 16, 2022

Fecal Microbiota Transplantation With Immune Checkpoint Inhibitors in Lung Cancer
Details : Rifaximin is a Antibiotic drug candidate, which is currently being evaluated in phase II clinical studies for the treatment of Lung Neoplasms.
Product Name : Undisclosed
Product Type : Antibiotic
Upfront Cash : Inapplicable
August 16, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
XIFAXAN (rifaximin) is a 2 week prescription treatment that provided up to 6 months of relief from IBS-D symptoms of abdominal pain and diarrhea in a clinical trial.
Lead Product(s): Rifaximin,Inapplicable
Therapeutic Area: Gastroenterology Brand Name: Xifaxan-Generic
Study Phase: UndisclosedProduct Type: Antibiotic
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable August 11, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Rifaximin,Inapplicable
Therapeutic Area : Gastroenterology
Highest Development Status : Undisclosed
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Privately Held Alvogen Announces Favorable Ruling on Patents Related to the Company’s Proposed G...
Details : XIFAXAN (rifaximin) is a 2 week prescription treatment that provided up to 6 months of relief from IBS-D symptoms of abdominal pain and diarrhea in a clinical trial.
Product Name : Xifaxan-Generic
Product Type : Antibiotic
Upfront Cash : Inapplicable
August 11, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
XIFAXAN® (rifaximin) 550 mg tablets are indicated for the reduction in risk of overt hepatic encephalopathy (HE) recurrence in adults and for the treatment of irritable bowel syndrome with diarrhea (IBS-D) in adults.
Lead Product(s): Rifaximin,Inapplicable
Therapeutic Area: Neurology Brand Name: Xifaxan
Study Phase: Approved FDFProduct Type: Antibiotic
Sponsor: Salix Pharmaceuticals
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable July 28, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Rifaximin,Inapplicable
Therapeutic Area : Neurology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Salix Pharmaceuticals
Deal Size : Inapplicable
Deal Type : Inapplicable
Bausch Health Provides Update Following Oral Order in XIFAXAN® Patent Litigation
Details : XIFAXAN® (rifaximin) 550 mg tablets are indicated for the reduction in risk of overt hepatic encephalopathy (HE) recurrence in adults and for the treatment of irritable bowel syndrome with diarrhea (IBS-D) in adults.
Product Name : Xifaxan
Product Type : Antibiotic
Upfront Cash : Inapplicable
July 28, 2022

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
82
PharmaCompass offers a list of Rifaximin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Rifaximin manufacturer or Rifaximin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Rifaximin manufacturer or Rifaximin supplier.
PharmaCompass also assists you with knowing the Rifaximin API Price utilized in the formulation of products. Rifaximin API Price is not always fixed or binding as the Rifaximin Price is obtained through a variety of data sources. The Rifaximin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Flonorm manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Flonorm, including repackagers and relabelers. The FDA regulates Flonorm manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Flonorm API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Flonorm manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Flonorm supplier is an individual or a company that provides Flonorm active pharmaceutical ingredient (API) or Flonorm finished formulations upon request. The Flonorm suppliers may include Flonorm API manufacturers, exporters, distributors and traders.
click here to find a list of Flonorm suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Flonorm DMF (Drug Master File) is a document detailing the whole manufacturing process of Flonorm active pharmaceutical ingredient (API) in detail. Different forms of Flonorm DMFs exist exist since differing nations have different regulations, such as Flonorm USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Flonorm DMF submitted to regulatory agencies in the US is known as a USDMF. Flonorm USDMF includes data on Flonorm's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Flonorm USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Flonorm suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Flonorm Drug Master File in Japan (Flonorm JDMF) empowers Flonorm API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Flonorm JDMF during the approval evaluation for pharmaceutical products. At the time of Flonorm JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Flonorm suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Flonorm Drug Master File in Korea (Flonorm KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Flonorm. The MFDS reviews the Flonorm KDMF as part of the drug registration process and uses the information provided in the Flonorm KDMF to evaluate the safety and efficacy of the drug.
After submitting a Flonorm KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Flonorm API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Flonorm suppliers with KDMF on PharmaCompass.
A Flonorm CEP of the European Pharmacopoeia monograph is often referred to as a Flonorm Certificate of Suitability (COS). The purpose of a Flonorm CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Flonorm EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Flonorm to their clients by showing that a Flonorm CEP has been issued for it. The manufacturer submits a Flonorm CEP (COS) as part of the market authorization procedure, and it takes on the role of a Flonorm CEP holder for the record. Additionally, the data presented in the Flonorm CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Flonorm DMF.
A Flonorm CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Flonorm CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Flonorm suppliers with CEP (COS) on PharmaCompass.
A Flonorm written confirmation (Flonorm WC) is an official document issued by a regulatory agency to a Flonorm manufacturer, verifying that the manufacturing facility of a Flonorm active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Flonorm APIs or Flonorm finished pharmaceutical products to another nation, regulatory agencies frequently require a Flonorm WC (written confirmation) as part of the regulatory process.
click here to find a list of Flonorm suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Flonorm as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Flonorm API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Flonorm as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Flonorm and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Flonorm NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Flonorm suppliers with NDC on PharmaCompass.
Flonorm Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Flonorm GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Flonorm GMP manufacturer or Flonorm GMP API supplier for your needs.
A Flonorm CoA (Certificate of Analysis) is a formal document that attests to Flonorm's compliance with Flonorm specifications and serves as a tool for batch-level quality control.
Flonorm CoA mostly includes findings from lab analyses of a specific batch. For each Flonorm CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Flonorm may be tested according to a variety of international standards, such as European Pharmacopoeia (Flonorm EP), Flonorm JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Flonorm USP).

