
1. 2-pyridine Carboxylic Acid
2. 2-pyridinecarboxylic Acid
3. Calcium Dipicolinate Trihydrate
4. Chromium Picolinate
5. Iron(iii) Picolinate
6. Picolinate
7. Picolinic Acid
8. Picolinic Acid, Hydrochloride
9. Picolinic Acid, Sodium Salt
10. Pyridine-2-carboxylic Acid
1. 17949-65-4
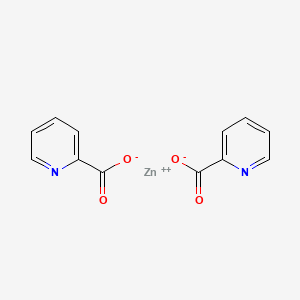
2. Zincpicolinate
3. Zinc;pyridine-2-carboxylate
4. Alo92o31se
5. Picolinic Acid Zinc Salt
6. Unii-alo92o31se
7. Zinc Pyridine-2-carboxylate
8. Dipicolinic Acid Zinc Salt
9. Schembl177833
10. Zinc Picolinate [inci]
11. Dtxsid60170814
12. Zinc Picolinate [who-dd]
13. Mfcd00145544
14. Akos015918238
15. Db11175
16. Ft-0652546
17. 2-pyridinecarboxylic Acid, Zinc Complex
18. A812451
19. J-011457
20. Q27273986
| Molecular Weight | 309.6 g/mol |
|---|---|
| Molecular Formula | C12H8N2O4Zn |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 0 |
| Exact Mass | 307.977549 g/mol |
| Monoisotopic Mass | 307.977549 g/mol |
| Topological Polar Surface Area | 106 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 108 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
Iron Chelating Agents
Organic chemicals that form two or more coordination links with an iron ion. Once coordination has occurred, the complex formed is called a chelate. The iron-binding porphyrin group of hemoglobin is an example of a metal chelate found in biological systems. (See all compounds classified as Iron Chelating Agents.)
