


1. Gs-9857
1. 1535212-07-7
2. Gs9857
3. Gs-9857
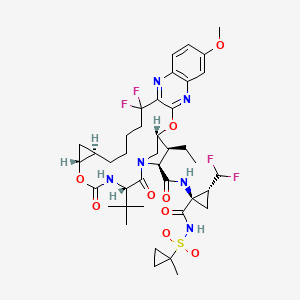
4. (1r,18r,20r,24s,27s,28s)-24-tert-butyl-n-[(1r,2r)-2-(difluoromethyl)-1-[(1-methylcyclopropyl)sulfonylcarbamoyl]cyclopropyl]-28-ethyl-13,13-difluoro-7-methoxy-22,25-dioxo-2,21-dioxa-4,11,23,26-tetrazapentacyclo[24.2.1.03,12.05,10.018,20]nonacosa-3,5(10),6,8,11-pentaene-27-carboxamide
5. 0570f37359
6. Voxilaprevir [inn]
7. Voxilaprevir [usan:inn]
8. Unii-0570f37359
9. Voxilaprevir [mi]
10. Voxilaprevir (usan/inn)
11. Voxilaprevir [usan]
12. Voxilaprevir [who-dd]
13. C40h52f4n6o9s
14. Chembl4474855
15. Schembl15412621
16. Dtxsid301027947
17. Voxilaprevir [orange Book]
18. Ex-a5390
19. Vosevi Component Voxilaprevir
20. Db12026
21. Voxilaprevir Component Of Vosevi
22. Hy-19840
23. Cs-0017027
24. J3.665.048f
25. D10899
26. Q27236086
27. (1ar,5s,8s,9s,10r,22ar)-5-tert-butyl-n-[(1 R,2r)-2-(difluoromethyl)-1-{[(1-methylcyclopropyl)sulfonyl]carbamoyl}cyclopropyl]-9-ethyl-18,18-difluoro-14- Methoxy-3,6-dioxo-1,1a,3,4,5,6,9,1 0,18,19,20,21,22,22a-tetradecahydro-8h-7,10-methanocyclopropa[18,19]
28. 8h-7,10-methanocyclopropa(18,19)(1,10,3,6)dioxadiazacyclononadecino(11,12-b)quinoxaline-8-carboxamide, N-((1r,2r)-2-(difluoromethyl)-1-((((1-methylcyclopropyl)sulfonyl)amino)carbonyl)cyclopropyl)-5-(1,1-dimethylethyl)-9-ethyl-18,18-difluoro-1,1a,3,4,5,6,9,10,18,19,20,21,22,22a-tetradecahydro-14-methoxy-3,6-dioxo-, (1ar,5s,8s,9s,10r,22ar)-
29. L9p
| Molecular Weight | 868.9 g/mol |
|---|---|
| Molecular Formula | C40H52F4N6O9S |
| XLogP3 | 5.9 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 15 |
| Rotatable Bond Count | 9 |
| Exact Mass | 868.34526108 g/mol |
| Monoisotopic Mass | 868.34526108 g/mol |
| Topological Polar Surface Area | 204 Ų |
| Heavy Atom Count | 60 |
| Formal Charge | 0 |
| Complexity | 1780 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Vosevi (Voxilaprevir/[DB08934]/[DB11613]) is approved for use in patients with genotypes 1-6 who have been previously treated with an NS5A inhibitor, or patients with genotypes 1a or 3 infection who have previously been treated with an HCV regimen containing [DB08934] without an NS5A inhibitor.
FDA Label
Voxilaprevir is a direct-acting antiviral agent that targets viral NS3/4A protein and causes a decrease in serum HCV RNA levels. It disrupts HCV replication by specifically inhibiting the critical functions of NS3/4A protein in the replication complex. It does not appear to prolong the QT interval even when given at 9 times the maximum recommended dose.
Absorption
When provided as the fixed dose combination product Vosevi with [DB08934] and [DB11613], voxilaprevir reaches a maximum concentration (Cmax) of 192 ng/mL at a maximum time (Tmax) of 4 hours post-dose.
Route of Elimination
Voxilaprevir is primarily eliminated via biliary excretion.
Voxilaprevir is primarily metabolized by Cytochrome P450 3A4 (CYP3A4) and to a lesser extent by CYP2C8 and CYP1A2.
33 hr
Voxilaprevir exerts its antiviral action by reversibley binding and inhibiting the NS3/4A serine protease of Hepatitis C Virus (HCV). Following viral replication of HCV genetic material and translation into a single polypeptide, Nonstructural Protein 3 (NS3) and its activating cofactor Nonstructural Protein 4A (NS4A) are responsible for cleaving genetic material into the following structural and nonstructural proteins required for assembly into mature virus: NS3, NS4A, NS4B, NS5A, and NS5B. By inhibiting viral protease NS3/4A, voxilaprevir therefore prevents viral replication and function.
