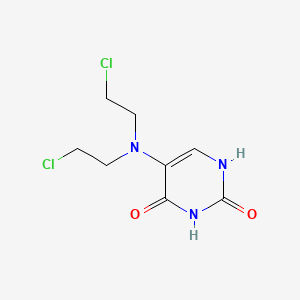

1. 5-(bis(2-chloroethyl)amino)-2,4-(1h,3h)pyrimidinedione
2. Mustard, Uracil
3. Uramustine
1. Uramustine
2. 66-75-1
3. Desmethyldopan
4. Aminouracil Mustard
5. Demethyldopan
6. Uramustin
7. Chlorethaminacil
8. Uracil Nitrogen Mustard
9. Uracilmostaza
10. Nordopan
11. Uracillost
12. 5-aminouracil Mustard
13. 5-bis(2-chloroethyl)aminouracil
14. 5-n,n-bis(2-chloroethyl)aminouracil
15. U-8344
16. Nsc-34462
17. 5-(di-2-chloroethyl)aminouracil
18. 5-[bis(2-chloroethyl)amino]uracil
19. Uracil Mustard [usan]
20. Ent 50439
21. Cb-4835
22. Rcra Waste Number U237
23. Nci-c04820
24. Sk-19849
25. 5-(bis(2-chloroethyl)amino)uracil
26. 2,6-dihydroxy-5-bis[2-chloroethyl]aminopyrimidine
27. 5-[di(beta-chloroethyl)amino]uracil
28. Uramustine (inn)
29. Uramustine [inn]
30. U 8344
31. 5-[bis(2-chloroethyl)amino]-1h-pyrimidine-2,4-dione
32. 5-(bis(2-chlorethyl)amino)-2,4(1h,3h)pyrimidinedione
33. Uracil Mustard (usan)
34. 5-(bis(2-chloroethyl)amino)-2,4(1h,3h)pyrimidinedione
35. 2,4(1h,3h)-pyrimidinedione, 5-(bis(2-chloroethyl)amino)-
36. 2,6-dihydroxy-5-bis(2-chloroethyl)aminopyrimidine
37. Uracil, 5-(bis(2-chloroethyl)amino)-
38. W7kq46gj8u
39. Chebi:9884
40. 5-[di(2-chloroethyl)amino]uracil
41. 5-[di(.beta.-chloroethyl)amino]uracil
42. Uracil, 5-[bis(2-chloroethyl)amino]-
43. 5-[bis(2-chloroethyl)amino]-2,4(1h,3h)-pyrimidinedione
44. Ncgc00160575-01
45. Uramustinum
46. 2,4(1h,3h)-pyrimidinedione, 5-[bis(2-chloroethyl)amino]-
47. Uramustina
48. Uracil Lost
49. Uracil Lost [german]
50. Uramustinum [inn-latin]
51. Uramustina [inn-spanish]
52. 5-(bis(2-chloroethyl)amino)pyrimidine-2,4(1h,3h)-dione
53. 5-[bis(2-chloroethyl)amino]pyrimidine-2,4(1h,3h)-dione
54. 5-(di(2-chloroethyl)amino)uracil
55. 5-(di-(beta-chloroethyl)amino)uracil
56. Ccris 618
57. Uracil Mustard (tn)
58. Hsdb 3261
59. Einecs 200-631-3
60. Nsc 34462
61. Rcra Waste No. U237
62. Unii-w7kq46gj8u
63. Uracil Mustard [usan:usp]
64. Uracil-mustard
65. Ai3-50439
66. 5-[bis(2-chloroethyl)amino]pyrimidine-2,4-diol
67. Dsstox_cid_6270
68. Schembl4091
69. Uramustine [mart.]
70. Chembl1488
71. Dsstox_rid_78084
72. Uracil Mustard [mi]
73. Uramustine [who-dd]
74. Dsstox_gsid_26270
75. Mls003899236
76. Uracil Mustard [hsdb]
77. Uracil Mustard [iarc]
78. Wln: T6mvmvj En2g2g
79. Uracil Mustard [vandf]
80. Gtpl7621
81. Zinc2235
82. Dtxsid8026270
83. Schembl19915183
84. Bcp27982
85. Nsc34462
86. Tox21_111911
87. Mfcd00233542
88. Uracil Mustard [orange Book]
89. Akos015850649
90. Akos024332500
91. Cs-5184
92. Db00791
93. Cas-66-75-1
94. Uracil, 5-[bis(2-chloroethyl)-amino]-
95. Hy-13544
96. Nci60_003061
97. Smr002543516
98. Db-005369
99. Ft-0602268
100. 5-(bis-(2-chloroethyl)-amino)-uracil
101. D06265
102. Ab01273928-01
103. 5-[bis(2-chlorethyl)amino]-2,3h)pyrimidinedione
104. A835541
105. 2,3h)-pyrimidinedione, 5-[bis(2-chloroethyl)amino]-
106. 2,6-dihydroxy-5-[bis(2-chloroethyl)amino]pyrimidine
107. Q15211075
108. Uracil Mustard (500 Mg) (for U.s. Sale Only)
109. 5-[bis(2-chloroethyl)amino]-1,2,3,4-tetrahydropyrimidine-2,4-dione
| Molecular Weight | 252.09 g/mol |
|---|---|
| Molecular Formula | C8H11Cl2N3O2 |
| XLogP3 | 0.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 5 |
| Exact Mass | 251.0228320 g/mol |
| Monoisotopic Mass | 251.0228320 g/mol |
| Topological Polar Surface Area | 61.4 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 288 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antineoplastic Agents, Alkylating
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
URACIL MUSTARD HAS BEEN TESTED IN LAB ANIMALS AS IMMUNOSUPRESSIVE AGENT & AGAINST INFLUENZA & VACCINIA VIRUSES & CERTAIN STRAINS OF BACTERIA.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V9 236 (1975)
IT MAY BE USED IN PALLIATIVE TREATMENT OF CHRONIC LYMPHOCYTIC LEUKEMIA, MALIGNANT LYMPHOMAS, & HODGKIN'S DISEASE. URACIL MUSTARD ALSO IS EFFECTIVE IN CHRONIC MYELOCYTIC LEUKEMIA & IMPROVEMENT HAS BEEN REPORTED IN OCCASIONAL CASES OF POLYCYTHEMIA VERA, MYCOSIS FUNGOIDES, & OVARIAN CARCINOMA.
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 1117
Uracil mustard has been used for the following indications, although use has generally been replaced by that of more effective agents: for palliative treatment of chronic lymphocytic and myelocytic leukemia; for palliative treatment of non-Hodgkin's lymphomas of the histiocytic or lymphocytic type; for palliative treatment of mycosis fungoides; for palliative treatment of the early stages of polycythemia vera before the development of leukemia or myelofibrosis. /Included in US product labeling/
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 2938
For more Therapeutic Uses (Complete) data for URACIL MUSTARD (6 total), please visit the HSDB record page.
AGENT IS CONTRAINDICATED IN PRESENCE OF PRONOUNCED LEUKOPENIA, THROMBOCYTOPENIA, OR APLASTIC ANEMIA & SHOULD NOT BE USED DURING FIRST TRIMESTER OF PREGNANCY TO AVOID POSSIBLE & POTENTIAL DAMAGING EFFECTS ON FETUS.
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 1117
SERUM URIC ACID LEVELS SHOULD BE MEASURED REGULARLY TO AVOID POSSIBLE HYPERURICEMIC NEPHROPATHY & ACUTE RENAL FAILURE. HEMATOPOIETIC EFFECTS ... ARE CUMULATIVE ... COMPLETE BLOOD CELL COUNTS SHOULD BE MADE ONCE WEEKLY DURING 1ST MO OF THERAPY ...
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 1117
EXTREME CAUTION IS INDICATED WHEN THERE IS DECR IN RBC COUNT OR HEMOGLOBIN LEVEL OF 30% OR MORE BELOW PRETREATMENT LEVEL. DRUG SHOULD BE WITHDRAWN IMMEDIATELY IF THERE IS SHARP FALL IN COUNT OF ANY OF FORMED ELEMENTS. AMBULATORY PT SHOULD NOT RECEIVE MORE THAN 1-WK SUPPLY OF DRUG @ ANY VISIT.
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 1117
URACIL MUSTARD SHOULD NOT BE ADMIN FOR SEVERAL WK AFTER COMPLETING COURSE OF TREATMENT WITH ANOTHER CYTOTOXIC DRUG OR RADIATION, SINCE IT IS ESSENTIAL TO ALLOW BONE MARROW FUNCTION TO RETURN TO SATISFACTORY LEVELS.
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 1117
For more Drug Warnings (Complete) data for URACIL MUSTARD (9 total), please visit the HSDB record page.
Used for its antineoplastic properties.
Uracil Mustard selectively inhibits the synthesis of deoxyribonucleic acid (DNA). The guanine and cytosine content correlates with the degree of Uracil Mustard-induced cross-linking. At high concentrations of the drug, cellular RNA and protein synthesis are also suppressed.
Antineoplastic Agents, Alkylating
A class of drugs that differs from other alkylating agents used clinically in that they are monofunctional and thus unable to cross-link cellular macromolecules. Among their common properties are a requirement for metabolic activation to intermediates with antitumor efficacy and the presence in their chemical structures of N-methyl groups, that after metabolism, can covalently modify cellular DNA. The precise mechanisms by which each of these drugs acts to kill tumor cells are not completely understood. (From AMA, Drug Evaluations Annual, 1994, p2026) (See all compounds classified as Antineoplastic Agents, Alkylating.)
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01A - Alkylating agents
L01AD - Nitrosoureas
L01AD08 - Uramustine
URACIL MUSTARD IS ABSORBED QUICKLY BUT NOT COMPLETELY AFTER ORAL ADMIN IN DOGS. CONCN IN PLASMA DECLINED AFTER EITHER ORAL (2 MG/KG) OR IV (1 MG/KG) ADMIN, & NO EVIDENCE OF DRUG WAS DETECTED @ 2 HR. LESS THAN 1% OF ADMIN DOSE WAS RECOVERED UNCHANGED IN URINE.
Gilman, A.G., L.S.Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 7th ed. New York: Macmillan Publishing Co., Inc., 1985., p. 1258
2-(14)C-URACIL MUSTARD WAS ADMIN @ DOSE OF 4 MG TO 265 WALKER CARCINOSARCOMA BEARING HOLZMAN RATS. INCORPORATION OF (14)C LABEL INTO MACROMOLECULES IN SUBCELLULAR FRACTIONS OF VARIOUS TISSUES WAS MEASURED FOR 6 HR AFTER ADMIN AND WAS GENERALLY FOUND TO BE MAXIMAL BY 1 HR & TO BE MORE EXTENSIVE IN RNA THAN IN DNA OR PROTEIN.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V9 239 (1975)
THE REACTION OF 0.2 UMOLES/ML URACIL MUSTARD WITH HEPARINIZED HUMAN BLOOD @ 37 C IN VITRO WAS MEASURED COLORIMETRICALLY: ABOUT 50% OR ORIGINAL DRUG WAS NO LONGER DETECTABLE AFTER 30 MIN.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V9 239 (1975)
After activation, it binds preferentially to the guanine and cytosine moieties of DNA, leading to cross-linking of DNA, thus inhibiting DNA synthesis and function.
... ITS ANTINEOPLASTIC EFFECTS ARE STRICTLY THOSE OF ITS BIS-CHLOROETHYLAMINE MOIETY.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1085
Uracil mustard, as an alkylating agent, interferes with DNA replication and transcription of RNA, and ultimately results in the disruption of nucleic acid function.
McEvoy G.K. (ed.). American Hospital Formulary Service-Drug Information 96. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1996 (Plus Supplements)., p. 795