


1. Actidil
2. Anhydrous, Triprolidine Hydrochloride
3. Hydrochloride Anhydrous, Triprolidine
4. Hydrochloride, Triprolidine
5. Pro Actidil
6. Triprolidine
7. Triprolidine Hydrochloride Anhydrous
8. Triprolidine Monohydrochloride
9. Triprolidine Monohydrochloride, (z)-isomer
10. Triprolidine Monohydrochloride, Monohydrate
11. Triprolidine Oxalate
12. Triprolidine Oxalate, (trans)-isomer
13. Triprolidine, (z)-isomer
1. 550-70-9
2. Actidilat
3. Venen
4. Actidil
5. Trans-triprolidine Hydrochloride
6. Pro-actidil
7. Pro-entra
8. Triprolidine Hydrochloride Anhydrous
9. 6138-79-0
10. Entra
11. (e)-2-(3-(pyrrolidin-1-yl)-1-(p-tolyl)prop-1-en-1-yl)pyridine Hydrochloride
12. Triprolidine Hydrochloride (anhydrous)
13. Mls000028751
14. Ng7a104r3j
15. Chebi:84119
16. Pyridine, 2-(1-(4-methylphenyl)-3-(1-pyrrolidinyl)-1-propenyl)-, Monohydrochloride, (e)-
17. Smr000058521
18. Triprolidine (hydrochloride)
19. Triprolidine Hydrochloride (anh.)
20. Sr-01000075292
21. Einecs 208-985-0
22. Unii-ng7a104r3j
23. Pyridine, 2-((1e)-1-(4-methylphenyl)-3-(1-pyrrolidinyl)-1-propenyl)-, Monohydrochloride
24. Prestwick_575
25. Mfcd00039044
26. Opera_id_1214
27. (e)-2-[3-(1-pyrrolidinyl)-1-p-tolylpropenyl]pyridine Hydrochloride
28. Trans-2-(3-(1-pyrrolidinyl)-1-p-tolylpropenyl)pyridine Monohydrochloride
29. Schembl42145
30. Mls001148254
31. Mls002207213
32. Mls002222226
33. Spectrum1500598
34. Chembl1200450
35. Hy-b1808a
36. Dtxsid10872513
37. Hms1568f06
38. Hms1921i07
39. Hms3411o04
40. Hms3675o04
41. Pharmakon1600-01500598
42. Triprolidine Hydrochloride, >=99%
43. Tox21_501130
44. Ccg-40331
45. Nsc757361
46. Triprolidine Monohydrochloride (anh.)
47. Akos015962154
48. Lp01130
49. Triprolidine Monohydrochloride Anhydrous
50. Pyridine, 2-(3-(1-pyrrolidinyl)-1-p-tolylpropenyl)-, Monohydrochloride, (e)-
51. Ncgc00094397-01
52. Ncgc00094397-02
53. Ncgc00094397-03
54. Ncgc00261815-01
55. Ac-15905
56. As-13971
57. Triprolidine Monohydrochloride (anhydrous)
58. Anhydrous Triprolidine Hydrochloride
59. Triprolidine Hydrochloride [who-dd]
60. Cs-0013855
61. Eu-0101130
62. T 6764
63. 138t790
64. A868718
65. A923663
66. Anhydrous Triprolidine Hydrochloride [mart.]
67. Sr-01000075292-1
68. Sr-01000075292-3
69. Sr-01000075292-7
70. Triprolidine Hydrochloride, Sigma Reference Standard
71. Q27157492
72. (e)-2-(3-(1-pyrrolidinyl)-1-p-tolylpropenyl)pyridine Monohydrochloride
73. (e)-2-(3-(pyrrolidin-1-yl)-1-(p-tolyl)prop-1-en-1-yl)pyridinehydrochloride
74. (e)-2-[1-(4-methylphenyl)-3-(1-pyrrolidinyl)-1-propenyl]pyridine Hydrochloride
75. 1-[(2e)-3-(4-methylphenyl)-3-(pyridin-2-yl)prop-2-en-1-yl]pyrrolidinium Chloride
76. 2-[(1e)-1-(4-methylphenyl)-3-(pyrrolidin-1-yl)prop-1-en-1-yl]pyridine Hydrochloride
| Molecular Weight | 314.9 g/mol |
|---|---|
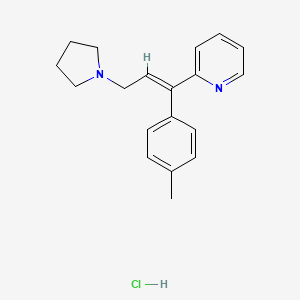
| Molecular Formula | C19H23ClN2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 4 |
| Exact Mass | 314.1549764 g/mol |
| Monoisotopic Mass | 314.1549764 g/mol |
| Topological Polar Surface Area | 16.1 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 336 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Histamine H1 Antagonists
Drugs that selectively bind to but do not activate histamine H1 receptors, thereby blocking the actions of endogenous histamine. Included here are the classical antihistaminics that antagonize or prevent the action of histamine mainly in immediate hypersensitivity. They act in the bronchi, capillaries, and some other smooth muscles, and are used to prevent or allay motion sickness, seasonal rhinitis, and allergic dermatitis and to induce somnolence. The effects of blocking central nervous system H1 receptors are not as well understood. (See all compounds classified as Histamine H1 Antagonists.)
Anti-Allergic Agents
Agents that are used to treat allergic reactions. Most of these drugs act by preventing the release of inflammatory mediators or inhibiting the actions of released mediators on their target cells. (From AMA Drug Evaluations Annual, 1994, p475) (See all compounds classified as Anti-Allergic Agents.)
