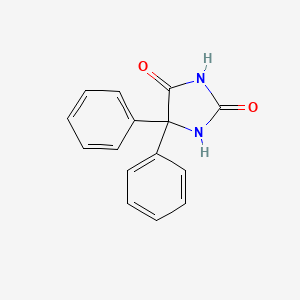

1. 5,5-diphenylhydantoin
2. 5,5-diphenylimidazolidine-2,4-dione
3. Antisacer
4. Difenin
5. Dihydan
6. Dilantin
7. Diphenylhydantoin
8. Diphenylhydantoinate, Sodium
9. Epamin
10. Epanutin
11. Fenitoin
12. Hydantol
13. Phenhydan
14. Phenytoin Sodium
15. Sodium Diphenylhydantoinate
1. 5,5-diphenylhydantoin
2. 57-41-0
3. Diphenylhydantoin
4. Dilantin
5. 5,5-diphenylimidazolidine-2,4-dione
6. Phenytoine
7. Epamin
8. Zentropil
9. Lepitoin
10. Dihydantoin
11. Aleviatin
12. Dilabid
13. Diphantoin
14. Diphenylan
15. Diphedan
16. Fenylepsin
17. Phentytoin
18. Sodanton
19. Difenin
20. Dihycon
21. Lehydan
22. Diphenylhydatanoin
23. Dantoinal
24. Di-hydan
25. Dilantine
26. Dillantin
27. Diphenine
28. Diphentyn
29. Ditoinate
30. Elepsindon
31. Epilantin
32. Fenitoina
33. Fenytoine
34. Hidantilo
35. Hidantina
36. Hidantomin
37. Hydantoinal
38. Kessodanten
39. Phanantin
40. Phanatine
41. Phenatoine
42. Sodantoin
43. Sylantoic
44. Thilophenyl
45. Zentronal
46. Auranile
47. Dantinal
48. Dantoine
49. Difetoin
50. Difhydan
51. Dintoin
52. Dintoina
53. Diphedal
54. Diphenin
55. Enkelfel
56. Epifenyl
57. Epihydan
58. Fentoin
59. Hidantal
60. Hydantal
61. Idantoil
62. Idantoin
63. Labopal
64. Phentoin
65. Ritmenal
66. Saceril
67. Sanepil
68. Silantin
69. Solantin
70. Danten
71. Denyl
72. Epelin
73. Epinat
74. Epised
75. Eptal
76. Hidan
77. Lepsin
78. Ekko
79. Ictalis Simple
80. Toin Unicelles
81. Dilantin Acid
82. Dantoinal Klinos
83. Om-hydantoine
84. Di-phetine
85. Epdantoine Simple
86. Hidantina Vitoria
87. Gerot-epilan-d
88. Epilan-d
89. Neosidantoina
90. Comitoina
91. Hidantina Senosian
92. Hydantol
93. Minetoin
94. Novantoina
95. Causoin
96. Convul
97. Di-lan
98. Ekko Capsules
99. Neos-hidantoina
100. 2,4-imidazolidinedione, 5,5-diphenyl-
101. Om Hidantoina Simple
102. Toin
103. Phenhydanin
104. Phenytex
105. Phenytoinum
106. Sinergina
107. Sodanthon
108. Iphenylhydantoin
109. Phenytoin-gerot
110. Difenilhidantoina
111. Fenytoin Dak
112. Didan Tdc 250
113. Dilantin-125
114. 5,5-diphenyl-2,4-imidazolidinedione
115. Epdantoin Simple
116. Phenytoin Awd
117. Epilan D
118. Diphenat
119. Hindatal
120. Hydantin
121. Epanutin
122. Fenitoina [inn-spanish]
123. Phenytoine [inn-french]
124. Phenytoinum [inn-latin]
125. Difenilhidantoina [spanish]
126. Diphenylhydantoine [french]
127. 5,5-dwufenylohydantoina
128. Antisacer
129. Fenantoin Mn Pharma
130. Diphenylhydantoine
131. Di-lan (van)
132. Phenytoin Sodium
133. Diphenylhydantoin (van)
134. Diphentoin
135. Dilantin-30
136. Solantoin
137. Solantyl
138. Eptoin
139. Dph (van)
140. 5,5-diphenylimidazolidin-2,4-dione
141. 5,5-diphenyl-imidazolidine-2,4-dione
142. 5,5-diphenylhydantoin (iupac)
143. Phenytek
144. 5,5-dwufenylohydantoina [polish]
145. Hydantoin, 5,5-diphenyl-
146. Ccris 515
147. Chebi:8107
148. 5,5-diphenyl Hydantoin
149. Nci-c55765
150. 5,5-diphenylhydantoin (phenytoin)
151. Diphenylan Sodium
152. Ai3-52498
153. Nsc-8722
154. Dilantin (tn)
155. Novophenytoin
156. Mls000069789
157. Citrulliamon
158. Phenitoin
159. 5,5-diphenyltetrahydro-1h-2,4-imidazoledione
160. Fenidantoin S
161. Nsc8722
162. Sm-88 Component Phenytoin
163. 6158tkw0c5
164. Epasmir 5
165. Ncgc00021139-03
166. Smr000059026
167. Dsstox_cid_541
168. Fenidantoin "s"
169. Dsstox_rid_75650
170. Dsstox_gsid_20541
171. Epasmir "5"
172. Didan-tdc-250
173. Cas-57-41-0
174. Phenytoin (phn)
175. Component Of Mebroin
176. Fenidantoin ''s''
177. Epasmir ''5''
178. Nsc 8722
179. 630-93-3
180. Einecs 200-328-6
181. Mfcd00005264
182. Unii-6158tkw0c5
183. Sr-01000075211
184. Iflab1_000214
185. Fenidantoin 's'
186. Hsdb 3160
187. Episar (salt/mix)
188. Epasmir '5'
189. Aladdin (salt/mix)
190. Alepsin (salt/mix)
191. Epsolin (salt/mix)
192. Phenytoin (lepitoin)
193. Tacosal (salt/mix)
194. Phenytoin [usan:usp:inn:ban:jan]
195. Antisacer (salt/mix)
196. Epdantoin (salt/mix)
197. Epileptin (salt/mix)
198. Hydantoin,5-diphenyl-
199. Spectrum_001105
200. Fenigramon (salt/mix)
201. Citrullamon (salt/mix)
202. Opera_id_394
203. Phenytoin [inn]
204. Phenytoin [jan]
205. 2, 5,5-diphenyl-
206. Phenytoin [mi]
207. Chembl16
208. Phenytoin [hsdb]
209. Phenytoin [iarc]
210. Phenytoin [usan]
211. Spectrum2_001281
212. Spectrum3_000890
213. Spectrum4_000984
214. Spectrum5_001369
215. Lopac-d-4007
216. Phenytoin [vandf]
217. Epitope Id:117723
218. Phenytoin [mart.]
219. D 4007
220. Phenytoin [usp-rs]
221. Phenytoin [who-dd]
222. Phenytoin [who-ip]
223. Schembl3440
224. Bidd:pxr0090
225. Lopac0_000329
226. Lopac0_000378
227. Oprea1_373280
228. Bspbio_001437
229. Kbiogr_001387
230. Kbioss_001585
231. Mls001074087
232. Mls002454401
233. Bidd:gt0625
234. Divk1c_000507
235. Soluble Phenytoin (salt/mix)
236. Spbio_001281
237. Phenytoin (jp17/usp/inn)
238. Gtpl2624
239. Phenytoin [orange Book]
240. 2-hydroxy-5,5-diphenyl-3,5-dihydro-4h-imidazol-4-one
241. Dtxsid8020541
242. Phenytoin [ep Monograph]
243. Phenytoin [usp Impurity]
244. Kbio1_000507
245. Kbio2_001585
246. Kbio2_004153
247. Kbio2_006721
248. Kbio3_001780
249. Phenytoin [usp Monograph]
250. Wln: T5mvmv Ehj Er& Er
251. 5,5-diphenylhydantoin, >=99%
252. Ninds_000507
253. Phenytoin 1.0 Mg/ml In Methanol
254. Hms1412j16
255. Hms1694o05
256. Hms1791h19
257. Hms1989h19
258. Hms2089e11
259. Hms2236j06
260. Hms3261k17
261. Hms3402h19
262. Hms3657o03
263. Phenytoinum [who-ip Latin]
264. Bcp05960
265. Hy-b0448
266. Hydantoin, 5,5-diphenyl- (8ci)
267. Zinc2510358
268. Tox21_110861
269. Tox21_202299
270. Tox21_300281
271. Tox21_500378
272. Ac-376
273. Bdbm50003655
274. Bdbm50101816
275. S2525
276. Stk058029
277. Stk182871
278. Stl454130
279. Akos000416887
280. Akos003245432
281. Tox21_110861_1
282. 5,5-diphenylimidazolidine-2,4-dione.
283. Ccg-104011
284. Ccg-221682
285. Db00252
286. Lp00378
287. Phenytoin 1000 Microg/ml In Methanol
288. 5,5-di(phenyl)imidazolidine-2,4-dione
289. Idi1_000507
290. Idi1_008433
291. Ncgc00015342-01
292. Ncgc00015342-02
293. Ncgc00015342-03
294. Ncgc00015342-04
295. Ncgc00015342-05
296. Ncgc00015342-06
297. Ncgc00015342-07
298. Ncgc00015342-08
299. Ncgc00015342-09
300. Ncgc00015342-10
301. Ncgc00015342-11
302. Ncgc00015342-12
303. Ncgc00021139-01
304. Ncgc00021139-02
305. Ncgc00021139-04
306. Ncgc00021139-05
307. Ncgc00021139-06
308. Ncgc00021139-07
309. Ncgc00021139-08
310. Ncgc00021139-09
311. Ncgc00021139-10
312. Ncgc00021139-11
313. Ncgc00091492-01
314. Ncgc00091492-02
315. Ncgc00091492-03
316. Ncgc00091492-04
317. Ncgc00091492-05
318. Ncgc00093810-01
319. Ncgc00093810-02
320. Ncgc00254135-01
321. Ncgc00259848-01
322. Ncgc00261063-01
323. 5,5-?diphenyl-?2,4-?imidazolidinedione
324. 5,5-diphenyl-1h-imidazolidine-2,4-dione
325. D0894
326. Eu-0100378
327. Ft-0667653
328. Ft-0699999
329. P-235
330. Sw203757-2
331. En300-16818
332. 5,5-diphenylimidazolidine-2,4-dione;phenytoin
333. C07443
334. D00512
335. E76094
336. 2,4-imidazolidinedione, 5,5-diphenyl- (9ci)
337. 5,5-diphenyl-1h-imidazole-2,4(3h,5h)-dione
338. Ab00374253-10
339. Ab00374253-11
340. Ab00374253_13
341. A831435
342. Q410400
343. Sr-01000003141
344. Sr-01000003141-8
345. Sr-01000075211-2
346. W-105468
347. Brd-k55930204-001-02-7
348. Brd-k55930204-236-11-0
349. Z56786458
350. 4-hydroxy-5,5-diphenyl-1,5-dihydro-2h-imidazol-2-one
351. F0020-1370
352. Phenytoin, European Pharmacopoeia (ep) Reference Standard
353. Phenytoin, United States Pharmacopeia (usp) Reference Standard
354. 5,5-diphenylhydantoin Solution, Drug Standard, 1.0 Mg/ml In Methanol
355. Phenytoin, Pharmaceutical Secondary Standard; Certified Reference Material
356. Phenytoin For System Suitability, European Pharmacopoeia (ep) Reference Standard
357. Phenytoin Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 252.27 g/mol |
|---|---|
| Molecular Formula | C15H12N2O2 |
| XLogP3 | 2.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 252.089877630 g/mol |
| Monoisotopic Mass | 252.089877630 g/mol |
| Topological Polar Surface Area | 58.2 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 350 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 12 | |
|---|---|
| Drug Name | Dilantin |
| PubMed Health | Phenytoin (By mouth) |
| Drug Classes | Antiarrhythmic, Group IB, Anticonvulsant |
| Drug Label | Phenytoin sodium is an antiepileptic drug. Phenytoin sodium is related to the barbiturates in chemical structure, but has a five-membered ring. The chemical name is sodium 5,5-diphenyl-2, 4-imidazolidinedione, having the following structural formula:... |
| Active Ingredient | Phenytoin; Phenytoin sodium |
| Dosage Form | Capsule; Tablet, chewable |
| Route | Oral |
| Strength | 100mg extended; 50mg; 30mg extended |
| Market Status | Prescription |
| Company | Pfizer Pharms; Parke Davis |
| 2 of 12 | |
|---|---|
| Drug Name | Dilantin-125 |
| PubMed Health | Phenytoin (By mouth) |
| Drug Classes | Antiarrhythmic, Group IB, Anticonvulsant |
| Drug Label | Dilantin (phenytoin) is related to the barbiturates in chemical structure, but has a five-membered ring. The chemical name is 5,5-diphenyl-2,4 imidazolidinedione, having the following structural formula:Each 5 ml of suspension contains 125 mg of phen... |
| Active Ingredient | Phenytoin |
| Dosage Form | Suspension |
| Route | Oral |
| Strength | 125mg/5ml |
| Market Status | Prescription |
| Company | Parke Davis |
| 3 of 12 | |
|---|---|
| Drug Name | Extended phenytoin sodium |
| PubMed Health | Phenytoin |
| Drug Classes | Antiarrhythmic, Group IB, Anticonvulsant |
| Drug Label | Phenytoin sodium is an antiepileptic drug. Phenytoin sodium is related to the barbiturates in chemical structure, but has a five-membered ring. The chemical name is sodium 5,5-diphenyl-2, 4-imidazolidinedione, having the following structural formula:... |
| Active Ingredient | Phenytoin sodium |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 200mg extended; 100mg extended; 300mg extended; 30mg extended |
| Market Status | Prescription |
| Company | Wockhardt; Amneal Pharms Ny; Sun Pharm Inds (in); Sun Pharm Inds; Taro; Wockhardt Usa; Mylan |
| 4 of 12 | |
|---|---|
| Drug Name | Phenytek |
| Active Ingredient | Phenytoin sodium |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 300mg extended; 200mg extended |
| Market Status | Prescription |
| Company | Mylan |
| 5 of 12 | |
|---|---|
| Drug Name | Phenytoin |
| Drug Label | Phenytoin sodium is an antiepileptic drug. Phenytoin sodium is related to the barbiturates in chemical structure, but has a five-membered ring. The chemical name is sodium 5,5-diphenyl-2, 4-imidazolidinedione, having the following structural formula:... |
| Active Ingredient | Phenytoin |
| Dosage Form | Suspension; Tablet, chewable |
| Route | Oral |
| Strength | 125mg/5ml; 50mg |
| Market Status | Prescription |
| Company | Mylan Pharms; Wockhardt; Taro; Vistapharm |
| 6 of 12 | |
|---|---|
| Drug Name | Phenytoin sodium |
| Drug Label | Phenytoin sodium is an antiepileptic drug. Phenytoin sodium is related to the barbiturates in chemical structure, but has a five-membered ring. The chemical name is sodium 5,5-diphenyl-2, 4-imidazolidinedione, having the following structural formula:... |
| Active Ingredient | Phenytoin sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 50mg/ml |
| Market Status | Prescription |
| Company | Hospira; Hikma Maple; X-gen Pharms; Luitpold |
| 7 of 12 | |
|---|---|
| Drug Name | Dilantin |
| PubMed Health | Phenytoin (By mouth) |
| Drug Classes | Antiarrhythmic, Group IB, Anticonvulsant |
| Drug Label | Phenytoin sodium is an antiepileptic drug. Phenytoin sodium is related to the barbiturates in chemical structure, but has a five-membered ring. The chemical name is sodium 5,5-diphenyl-2, 4-imidazolidinedione, having the following structural formula:... |
| Active Ingredient | Phenytoin; Phenytoin sodium |
| Dosage Form | Capsule; Tablet, chewable |
| Route | Oral |
| Strength | 100mg extended; 50mg; 30mg extended |
| Market Status | Prescription |
| Company | Pfizer Pharms; Parke Davis |
| 8 of 12 | |
|---|---|
| Drug Name | Dilantin-125 |
| PubMed Health | Phenytoin (By mouth) |
| Drug Classes | Antiarrhythmic, Group IB, Anticonvulsant |
| Drug Label | Dilantin (phenytoin) is related to the barbiturates in chemical structure, but has a five-membered ring. The chemical name is 5,5-diphenyl-2,4 imidazolidinedione, having the following structural formula:Each 5 ml of suspension contains 125 mg of phen... |
| Active Ingredient | Phenytoin |
| Dosage Form | Suspension |
| Route | Oral |
| Strength | 125mg/5ml |
| Market Status | Prescription |
| Company | Parke Davis |
| 9 of 12 | |
|---|---|
| Drug Name | Extended phenytoin sodium |
| PubMed Health | Phenytoin |
| Drug Classes | Antiarrhythmic, Group IB, Anticonvulsant |
| Drug Label | Phenytoin sodium is an antiepileptic drug. Phenytoin sodium is related to the barbiturates in chemical structure, but has a five-membered ring. The chemical name is sodium 5,5-diphenyl-2, 4-imidazolidinedione, having the following structural formula:... |
| Active Ingredient | Phenytoin sodium |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 200mg extended; 100mg extended; 300mg extended; 30mg extended |
| Market Status | Prescription |
| Company | Wockhardt; Amneal Pharms Ny; Sun Pharm Inds (in); Sun Pharm Inds; Taro; Wockhardt Usa; Mylan |
| 10 of 12 | |
|---|---|
| Drug Name | Phenytek |
| Active Ingredient | Phenytoin sodium |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 300mg extended; 200mg extended |
| Market Status | Prescription |
| Company | Mylan |
| 11 of 12 | |
|---|---|
| Drug Name | Phenytoin |
| Drug Label | Phenytoin sodium is an antiepileptic drug. Phenytoin sodium is related to the barbiturates in chemical structure, but has a five-membered ring. The chemical name is sodium 5,5-diphenyl-2, 4-imidazolidinedione, having the following structural formula:... |
| Active Ingredient | Phenytoin |
| Dosage Form | Suspension; Tablet, chewable |
| Route | Oral |
| Strength | 125mg/5ml; 50mg |
| Market Status | Prescription |
| Company | Mylan Pharms; Wockhardt; Taro; Vistapharm |
| 12 of 12 | |
|---|---|
| Drug Name | Phenytoin sodium |
| Drug Label | Phenytoin sodium is an antiepileptic drug. Phenytoin sodium is related to the barbiturates in chemical structure, but has a five-membered ring. The chemical name is sodium 5,5-diphenyl-2, 4-imidazolidinedione, having the following structural formula:... |
| Active Ingredient | Phenytoin sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 50mg/ml |
| Market Status | Prescription |
| Company | Hospira; Hikma Maple; X-gen Pharms; Luitpold |
MEDICATION (VET): PHENYTOIN IS USED IN VETERINARY MEDICINE TO CONTROL EPILEPTIFORM CONVULSIONS IN DOGS. PHENYTOIN SODIUM HAS ALSO BEEN RECOMMENDED AS ANTICONVULSANT FOR DOGS.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V13 206
MEDICATION (VET): ...IS DRUG OF CHOICE IN DIGITALIS INDUCED TOXICITY & VENTRICULAR ARRHYTHMIAS IN DOGS UNRESPONSIVE TO PROCAINE AMIDE. IN CASES OF POOR WOUND HEALING IT MAY STIMULATE COLLAGEN FORMATION & WOUND TENSILE STRENGTH.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 185
Hydantoin anticonvulsants are indicated in the suppression and control of tonic-clonic (grand mal) and simple or complex partial (psychomotor or temporal lobe) seizures. /Hydantoin anticonvulsants; Included in US product labeling./
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 259
Parenteral phenytoin is indicated for the control of tonic-clonic type status epilepticus. Although parenteral benzodiazepines are often used initially for rapid control of status epilepticus, phenytoin is indicated for sustained control of seizure activity. /Included in US product labeling./
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 259
For more Therapeutic Uses (Complete) data for PHENYTOIN (11 total), please visit the HSDB record page.
Hydantoin anticonvulsants cross the placenta; risk-benefit must be considered, although a definite cause and effect relationship has not been established between the hydantoins and teratogenic effects. Reports in recent years indicate a higher incidence of congenital abnormalities in children whose mothers used anticonvulsant medication during pregnancy, although most epileptic mothers have delivered normal babies. Reported abnormalities include cleft lip, cleft palate, heart malformations, and the "fetal hydantoin syndrome" (characterized by prenatal growth deficiency, microcephaly, craniofacial abnormalities, hypoplasia of the fingernails, and mental deficiency assoc with intrauterine development during therapy). Medication has not been definitively proven to be the cause of "fetal hydantoin syndrome." The reports, to date, relate primarily to the more widely used anticonvulsants, phenytoin and phenobarbital. Pending availability of more precise info, this risk-benefit consideration of anticonvulsant use during pregnancy is extended to the entire family of anticonvulsant medications. /Hydantoin anticonvulsants/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 261
Because of altered absorption and protein binding and/or increased metabolic clearance of hydantoin anticonvulsants during pregnancy, pregnant women receiving these medications may experience an increased incidence of seizures. Serum hydantoin concentrations must be monitored and doses increased accordingly. A gradual resumption of the patient's usual dosage may be necessary after delivery. However, some patients may experience a rapid reduction in maternal hepatic phenytoin metabolism at time of delivery, requiring the dosage to be reduced within 12 hr postpartum. /Hydantoin anticonvulsants/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 261
Exposure to hydantoins prior to delivery may lead to an increased risk of life-threatening hemorrhage in the neonate, usually within 24 hr of birth. Hydantoins may also produce a deficiency of vitamin K in the mother, causing increased maternal bleeding during delivery. risk of maternal and infant bleeding may be reduced by administering water-soluble vitamin K to the mother during delivery and to the neonate, intramuscularly or sc, immediately after birth. /Hydantoin anticonvulsants/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 261
Phenytoin are distributed into breast milk; significant amounts may be ingested by the infant.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 261
For more Drug Warnings (Complete) data for PHENYTOIN (26 total), please visit the HSDB record page.
The lethal dose of phenytoin in adults is estimated to be 2 to 5 g. The lethal dose in children in unknown.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 266
Phenytoin is indicated to treat grand mal seizures, complex partial seizures, and to prevent and treat seizures during or following neurosurgery. Injectable phenytoin and [Fosphenytoin], which is the phosphate ester prodrug formulation of phenytoin, are indicated to treat tonic-clonic status epilepticus, and for the prevention and treatment of seizures occurring during neurosurgery.
Phenytoin is an anticonvulsant with a narrow therapeutic index. Although the recommended therapeutic range is cited to be between 10-20 mg/L, differences in albumin levels, genetics, comorbidities, and body composition can make achieving an ideal phenytoin dose challenging. For example, studies have confirmed that phenytoin metabolism is impacted by CYP2C9 genotype polymorphisms and possibly by CYP2C19 genotype polymorphisms (the latter has not been as extensively studied). It is worth nothing that although phenytoin is highly protein bound, only the fraction unbound is able to exert a pharmacological effect. Therefore, factors that reduce or increase the percentage of protein bound phenytoin (for example: concomitant administration of drugs that can cause displacement from protein binding sites) can have a marked impact on phenytoin therapy.
Anticonvulsants
Drugs used to prevent SEIZURES or reduce their severity. (See all compounds classified as Anticonvulsants.)
Cytochrome P-450 CYP1A2 Inducers
Drugs and compounds that induce the synthesis of CYTOCHROME P-450 CYP1A2. (See all compounds classified as Cytochrome P-450 CYP1A2 Inducers.)
Voltage-Gated Sodium Channel Blockers
A class of drugs that inhibit the activation of VOLTAGE-GATED SODIUM CHANNELS. (See all compounds classified as Voltage-Gated Sodium Channel Blockers.)
N03AB02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N03 - Antiepileptics
N03A - Antiepileptics
N03AB - Hydantoin derivatives
N03AB02 - Phenytoin
Absorption
Given its narrow therapeutic index, therapeutic drug monitoring is recommended to help guide dosing. Phenytoin is completely absorbed. Peak plasma concentration is attained approximately 1.5-3 hours, and 4-12 hours after administration of the immediate release formulation and the extended release formulation, respectively. It should be noted that absorption can be markedly prolonged in situations of acute ingestion.
Route of Elimination
The majority of phenytoin is excreted as inactive metabolites in the bile. An estimated 1-5% of phenytoin is eliminated unchanged in the urine.
Volume of Distribution
The volume of distribution of phenytoin is reported to be approximately 0.75 L/kg.
Clearance
The clearance of phenytoin is non-linear. At lower serum concentrations (less than 10 mg/L), elimination is characterized by first order kinetics. As plasma concentrations increase, the kinetics shift gradually towards zero-order, and finally reach zero-order kinetics once the system is saturated.
Studies using Dilantin have shown that phenytoin and its sodium salt are usually completely absorbed from the GI tract. Bioavailability may vary enough among oral phenytoin sodium preparations of different manufacturers to result in toxic serum concentrations or a loss of seizure control (subtherapeutic serum concentrations)...
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2217
Absorption of phenytoin is slow and variable among products (poor in neonates) for oral admininstration, immediate for iv administration, and very slow but complete (92%) for intramuscular administration.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 260
Prompt phenytoin capsules are rapidly absorbed and generally produce peak serum concentrations in 1.5-3 hours, while extended phenytoin sodium capsules are more slowly absorbed and generally produce peak serum concentrations in 4-12 hours. When phenytoin sodium is administered im, absorption may be erratic; this may result from crystallization of the drug at the injection site because of the change in pH.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2217
/Phenytoin/ is distributed into cerebrospinal fluid, saliva, semen, GI fluids, bile, and breast milk; it also crosses the placenta, with fetal serum concentrations equal to those of the mother.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 260
For more Absorption, Distribution and Excretion (Complete) data for PHENYTOIN (15 total), please visit the HSDB record page.
Phenytoin is extensively metabolized and is first transformed into a reactive _arene oxide intermediate_. It is thought that this reactive intermediate is responsible for many undesirable phenytoin adverse effects such as hepatotoxicity, SJS/TEN, and other idiosyncratic reactions. The _arene oxide_ is metabolized to either a _hydroxyphenytoin_ or _phenytoin dihydrodiol_ metabolite, although the former accounts for about 90% of phenytoin metabolism. Interestingly, two stereoisomers of the _hydroxyphenytoin_ metabolite are formed by CYP2C9 and CYP2C19: _(R)-p-HPPH_ and _(S)-p-HPPH_. When CYP2C19 catalyzes the reaction, the ratio of stereoisomers is roughly 1:1, whereas when CYP2C9 catalyzes the reaction, the ratio heavily favours the "S" stereoisomer. Since the metabolism of phenytoin is in part influenced by genetic polymorphisms of CYP2C9 and CYP2C19, this ratio can be utilized to identify different genomic variants of the enzymes. EPHX1, CYP1A2, CYP2A6, CYP2C19, CYP2C8, CYP2C9, CYP2D6, CYP2E1 and CYP3A4 are responsible for producing the _phenytoin dihydrodiol_ metabolite. _Hydroxyphenytoin_ can be metabolized by CYP2C19, CYP3A5, CYP2C9, CYP3A4, CYP3A7, CYP2B6 and CYP2D6 to a _phenytoin catechol_ metabolite or undergo glucuronidation by UGT1A6, UGT1A9, UGT1A1, and UGT1A4 to a _glucuronide metabolite_ that can be eliminated in the urine. On the other hand, the _phenytoin dihydrodiol_ entity is only transformed to the _catechol_ metabolite. The _catechol metabolite_ can undergo methylation by COMT and be subsequently eliminated in the urine, or can spontaneously oxidize to a _phenytoin quinone_ (NQO1 can transform the quinone back to the catechol metabolite). Of note, although CYP2C18 is poorly expressed in the liver, the enzyme is active in the skin and is involved in the primary and secondary hydroxylation of phenytoin. This CYP2C18 mediated bioactivation may be linked to the manifestation of adverse cutaneous drug reactions associated with phenytoin.
The major route of metabolism of phenytoin is oxidation by the liver to the inactive metabolite 5-(p-hydroxyphenyl)-5-phenylhydantoin (HPPH). Because this metabolism is a saturable process, small increases in dosage may produce substantial increases in plasma phenytoin concentrations...
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2217
The rate of hepatic biotransformation is increased in younger children, in pregnant women, in women during menses, and in patients with acute trauma; rate decreases with advancing age. The major inactive metabolite of phenytoin is 5-(p-hydroxyphenyl)-5-phenylhydantoin (HPPH). Phenytoin may be metabolized slowly in a small number of individuals due to genetic predisposition, which may cause limited enzyme availability and lack of induction.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 260
... Oxidative metabolism of 1 of geminal phenyl rings of diphenylhydantoin ... 5-meta-hydroxyphenyl-(l) and 5-para-hydroxyphenyl-5-phenylhydantoin were detected in urine of man (approx ratio 1:12) ...
Testa, B. and P. Jenner. Drug Metabolism: Chemical & Biochemical Aspects. New York: Marcel Dekker, Inc., 1976., p. 48
Phenytoin has known human metabolites that include (2S,3S,4S,5R)-6-(2,5-dioxo-4,4-diphenylimidazolidin-1-yl)-3,4,5-trihydroxyoxane-2-carboxylic acid, 3'-HPPH, 4-Hydroxyphenytoin, and 5-(3,4-dihydroxycyclohexa-1,5-dien-1-yl)-5-phenylimidazolidine-2,4-dione.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Oral administration: The half-life of phenytoin ranges from 7 to 42 hours, and is 22 hours on average. Intravenous administration: The half-life of phenytoin ranges from 10-15 hours.
Following oral administration, the plasma half-life of phenytoin averages about 22 hours, although the half-life has ranged from 7-42 hours in individual patients. The plasma half-life of phenytoin in humans following IV administration ranges from 10-15 hours.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2217
Because phenytoin exhibits saturable, zero-order, or dose-dependent pharmacokinetics, the apparent half-life of phenytoin changes with dose and serum concentrations. this is due to the saturation of the enzyme system responsible for metabolizing phenytoin, which occurs at therapeutic concentrations of the drug. Thus, a constant amount of drug is metabolized (capacity-limited metabolism), and small increases in dose may cause disproportionately large increases in serum concentrations and apparent half-life, possibly causing unexpected toxicity.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 260
Although phenytoin first appeared in the literature in 1946, it has taken decades for the mechanism of action to be more specifically elucidated. Although several scientists were convinced that phenytoin altered sodium permeability, it wasnt until the 1980s that this phenomenon was linked to voltage-gated sodium channels. Phenytoin is often described as a non-specific sodium channel blocker and targets almost all voltage-gated sodium channel subtypes. More specifically, phenytoin prevents seizures by inhibiting the positive feedback loop that results in neuronal propagation of high frequency action potentials.
The mechanism of action is not completely known, but it is thought to involve stabilization of neuronal membranes at the cell body, axon, and synapse and limitation of the spread of neuronal or seizure activity. In neurons, phenytoin decreases sodium and calcium ion influx by prolonging channel inactivation time during generation of nerve impulses. Phenytoin blocks the voltage-dependant sodium channels of neurons and inhibits the calcium flux across neuronal membranes, thus helping to stabilize neurons. It also decreases synaptic transmission, and decreases post-tetanic potentiation at the synapse. Phenytoin enhances the sodium ATPase activity of neurons and/or glial cells. It also influences second messenger systems by inhibiting calcium-calmodulin protein phosphorylation and possibly altering cyclic nucleotide production or metabolism.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 259
Phenytoin may act to normalize influx of sodium and calcium to cardiac Purkinje fibers. Abnormal ventricular automaticity and membrane responsiveness are decreased. Also, phenytoin shortens the refractory period, and therefore shortens the QT interval and the duration of the action potential.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 259
Exact mechanism is unknown. Phenytoin may act in the CNS to decrease synaptic transmission or to decrease summation of temporal stimulation leading to neuronal discharge (antikindling). Phenytoin raises the threshold of facial pain and shortens the duration of attacks by diminishing self-maintenance of excitation and repetitive firing.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 259
Phenytoin's mechanisms of action as a muscle relaxant is thought to be similar to its anticonvulsant action. In movement disorders, the membrane stabilizing effect reduces abnormal sustained repetitive firing and potentiation of nerve and muscle cells.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 260
A number of studies suggest that keratinocyte growth factor (KGF) plays a major part in reepithelialization after injury, via binding to the specific KGF receptor (KGFR). Several pharmacological agents, including the anti-epileptic drug phenytoin (PHT), have been widely used clinically to promote wound healing. Although the mechanism of action of PHT in this process is still not well understood, it is possible that the activity of PHT in wound healing is mediated via KGF and the KGFR. In the present study, using the enzyme-linked immunosorbant assay and flow cytometry we have shown that PHT increases KGF secretion and KGFR expression by more than 150% in gingival fibroblasts and epithelial cells, respectively. Moreover, semi-quantitative reverse transcriptase-polymerase chain reaction analysis showed that PHT also markedly increased both KGF and KGFR gene transcription by these cells.
PMID:11352631 Das S et al; Biochem Biophys Res Commun 282 (4): 875-81(2001)