


1. Pentapeptide, Thymopoietin
2. Thymopentin
3. Timunox
4. Tp-5
1. 69558-55-0
2. Ncgc00166412-01
3. Specplus_000687
4. Spectrum4_001271
5. Arg-lys-asp-val-tyr-oh
6. Thymopentin Acetate(tp-5)
7. Thymopoietin Ii-32-36)
8. Kbiogr_001882
9. Divk1c_006783
10. Schembl989519
11. Chembl1868258
12. Kbio1_001727
13. Dtxsid10860899
14. Hms3369g09
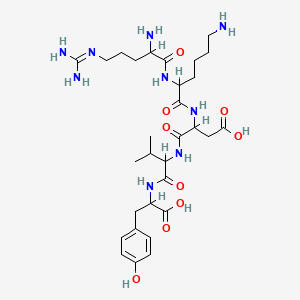
15. (3s)-3-[[(2s)-6-amino-2-[[(2s)-2-amino-5-(diaminomethylideneamino)pentanoyl]amino]hexanoyl]amino]-4-[[(2s)-1-[[(1s)-1-carboxy-2-(4-hydroxyphenyl)ethyl]amino]-3-methyl-1-oxobutan-2-yl]amino]-4-oxobutanoic Acid
16. Nsc645363
17. Akos015961651
18. Akos026750555
19. Ac-8959
20. Nsc-645363
21. As-13028
22. Dl-arginyl-dl-lysyl-dl-aspartyl-dl-valyl-dl-tyrosine
23. J-011348
24. N~5~-(diaminomethylidene)ornithyllysyl-alpha-aspartylvalyltyrosine
25. L-tyrosine, N-[n-[n-(n2-l-arginyl-l-lysly)-l-.alpha.-aspartyl]-l-valyl]-
| Molecular Weight | 679.8 g/mol |
|---|---|
| Molecular Formula | C30H49N9O9 |
| XLogP3 | -6.4 |
| Hydrogen Bond Donor Count | 11 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 22 |
| Exact Mass | 679.36532417 g/mol |
| Monoisotopic Mass | 679.36532417 g/mol |
| Topological Polar Surface Area | 328 Ų |
| Heavy Atom Count | 48 |
| Formal Charge | 0 |
| Complexity | 1110 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 5 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Adjuvants, Immunologic
Substances that augment, stimulate, activate, potentiate, or modulate the immune response at either the cellular or humoral level. The classical agents (Freund's adjuvant, BCG, Corynebacterium parvum, et al.) contain bacterial antigens. Some are endogenous (e.g., histamine, interferon, transfer factor, tuftsin, interleukin-1). Their mode of action is either non-specific, resulting in increased immune responsiveness to a wide variety of antigens, or antigen-specific, i.e., affecting a restricted type of immune response to a narrow group of antigens. The therapeutic efficacy of many biological response modifiers is related to their antigen-specific immunoadjuvanticity. (See all compounds classified as Adjuvants, Immunologic.)
