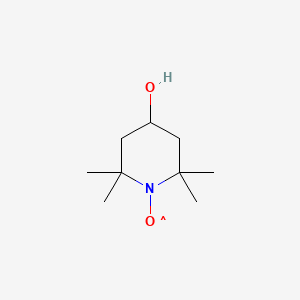

1. 2,2,6,6-tetramethyl-4-piperidinol-n-oxyl
2. 4-hydroxy-1-oxyl-2,2,6,6-tetramethylpiperidine
3. 4-hydroxy-2,2,6,6-tetramethylpiperidine-n-oxyl
4. 4-hydroxy-2,2,6,6-tetramethylpiperidinoxy Radical
5. 4-hydroxy-2,2,6,6-tetramethylpiperidinyl-1-oxy
6. Hytempo
7. N-oxyl-2,2,6,6-tetramethylpiperidine
8. Nitroxide 4-hydroxy-2,2,6,6-tetramethylpiperidinyl-n-oxyl
9. Nitroxyl-2 (2,2,6,6-tetramethyl 4-oxypiperidine)-1-oxyl
10. Tanol
11. Tmpn
1. 4-oxypiperidol
2. 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl
3. 4-hydroxy-2,2,6,6-tetramethyl Piperidinyloxy
4. 4-hydroxy-2,2,6,6-tetramethyl-piperidinooxy
5. 4-hydroxytempo
6. 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl
7. 4-hydroxy-tempo, Free Radical
8. 4-hydroxy-2,2,6,6-tetramethylpiperidinooxy
9. Mbm-02
10. U78zx2f65x
11. 2,2,6,6-tetramethyl-4-hydroxypiperidinooxy
12. 4-hydroxy-2,2,6,6-tetramethylpiperidinooxyl
13. Spj-701
14. 4-hydroxy-2,2,6,6-tetramethylpiperidine-n-oxyl
15. 2,2,6,6-tetramethyl-4-hydroxypiperidinooxy Radical
16. Zj-701
17. 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-yl)oxidanyl
18. Nsc-142784
19. Piperidinooxy, 4-hydroxy-2,2,6,6-tetramethyl-
20. Hytempo
21. Ccris 4555
22. Nr 1
23. 2,2,6,6-tetramethyl-4-piperidinol-n-oxyl
24. 4-hydroxy Tempo, Free Radical
25. Einecs 218-760-9
26. Nsc 142784
27. Tempol [who-dd]
28. Tempol [mi]
29. 4-oh-tempo
30. 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl
31. Dsstox_cid_21280
32. Dsstox_rid_79678
33. Unii-u78zx2f65x
34. Dsstox_gsid_41280
35. 4-hydroxy-tempo, 97%
36. Chembl607023
37. Piperidinoxy,4-hydroxy-2,2,6,6-tetramethyl, Sebacate
38. Dtxsid4041280
39. Hsdb 8014
40. Chebi:180664
41. Hms3884n22
42. Tox21_300872
43. S2910
44. Akos015908135
45. Ccg-266372
46. Cs-6034
47. Db12449
48. Ncgc00248196-01
49. Ncgc00248196-02
50. Ncgc00254776-01
51. As-12539
52. Cas-2226-96-2
53. Hy-100561
54. B2046
55. Ft-0618621
56. H0865
57. 4-hydroxy-2,2,6,6-tetramethylpiperidinyloxy
58. 4-hydroxy-2,2,6,6-tetramethylpiperidinyloxyl
59. 26h962
60. 4-hydroxy-tempo, Purum, >=97.0% (chn)
61. Ec 218-760-9
62. 1-piperidinyloxy, 4-hydroxy-2,2,6,6-tetramethyl
63. 4-hydroxy-2,2,6,6-tetramethyl-piperidin-1-oxyl
64. 4-hydroxy-2,2,6,6-tetramethylpiperidinlyoxy
65. 1-piperidinyloxy,4-hydroxy-2,2,6,6-tetramethyl-
66. 4-hydroxy-2,2,6,6-tetramethyl Piperidinyloxy Free Radical
67. 4-hydroxy-2,2,6,6-tetramethyl Piperidinyloxy Freeradical
68. 4-hydroxy-2,2,6,6-tetramethyl Piperidinyloxyfree Radical
69. 4-hydroxy-2,2,6,6-tetramethylpiperidiney-1-oxyl
70. 4-hydroxy-2,2,6,6-tetramethylpiperidinyloxy, Free Radical
71. 4-hydroxy-2,2,6,6-tetramethyl-1-piperidinyloxy, Free Radical
72. 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl Free Radical
73. 2,2,6,6-tetramethyl-4-hydroxypiperidine 1-oxide Radical
| Molecular Weight | 172.24 g/mol |
|---|---|
| Molecular Formula | C9H18NO2 |
| XLogP3 | 0.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 172.133753817 g/mol |
| Monoisotopic Mass | 172.133753817 g/mol |
| Topological Polar Surface Area | 24.5 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 159 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Complete alopecia is a universal complication of whole brain radiation therapy which contributes to patient anxiety over treatment. Tempol, a nitroxide radioprotector, has been shown to protect against radiation-induced alopecia in an animal model. This phase Ib study was designed to evaluate the safety and side effect profile of topical Tempol in patients with brain metastases being treated with whole brain radiotherapy. Twelve patients with metastatic cancer to the brain were enrolled in the study between October 2000 and February 2003. Tempol (70 mg/mL concentration solution) was applied topically to the scalp 15 minutes before and washed off immediately after the completion of each of 10 fractions of whole brain radiation. Pharmacokinetic studies to evaluate the systemic absorption of Tempol were performed. Patients were assessed for toxicity before, during, and after Tempol administration. A secondary end point of the study, hair retention, was also scored. Eleven patients were treated with topical Tempol. Adverse events that were considered possibly, probably, or definitely related to Tempol, included asymptomatic grade 2 (two patients) and grade 1 (one patient) hypoglycemia, grade 1 forehead skin redness (one patient), grade 1 dry scalp (one patient), and grade 1 tingling sensation on the scalp (one patient). Tempol was not detected in blood samples from more than 50% of the patients. Mean maximum Tempol levels for individual patients at any time point varied from 0.4 to 3.1 umol/L. Hair retention was localized to the base of the scalp where the Tempol solution pooled after application in the first four patients on the study. Subsequently, full scalp hair retention was seen in three of final five evaluable patients after gauze had been wrapped around the head to hold the solution against the scalp. This study demonstrates that topical application of Tempol to the scalp before whole brain radiation is safe and well tolerated. Evidence of protection against radiation-induced alopecia was observed. A phase II study that uses a gel formulation to increase the exposure of scalp to Tempol has been initiated.
PMID:15475427 Metz JM et al; Clin Cancer Res 10 (19): 6411-7 (2004)
Neuroprotective Agents
Drugs intended to prevent damage to the brain or spinal cord from ischemia, stroke, convulsions, or trauma. Some must be administered before the event, but others may be effective for some time after. They act by a variety of mechanisms, but often directly or indirectly minimize the damage produced by endogenous excitatory amino acids. (See all compounds classified as Neuroprotective Agents.)
Protein Synthesis Inhibitors
Compounds which inhibit the synthesis of proteins. They are usually ANTI-BACTERIAL AGENTS or toxins. Mechanism of the action of inhibition includes the interruption of peptide-chain elongation, the blocking the A site of ribosomes, the misreading of the genetic code or the prevention of the attachment of oligosaccharide side chains to glycoproteins. (See all compounds classified as Protein Synthesis Inhibitors.)
Antioxidants
Naturally occurring or synthetic substances that inhibit or retard oxidation reactions. They counteract the damaging effects of oxidation in animal tissues. (See all compounds classified as Antioxidants.)
Radiation-Protective Agents
Drugs used to protect against ionizing radiation. They are usually of interest for use in radiation therapy but have been considered for other purposes, e.g. military. (See all compounds classified as Radiation-Protective Agents.)
Metabolism of different nitroxides with piperidine structure used as spin labels in electron spin resonance (ESR) studies in vitro and in vivo was investigated in human keratinocytes of the cell line HaCaT by GC and GC-MS technique combined with S-band ESR. Besides the well known reduction of the nitroxyl radicals to the ESR silent hydroxylamines as primary products our results indicate the formation of the corresponding secondary amines. These reductions are inhibited by the thiol blocking agent N-ethylmaleimide and by the strong inhibitors of the thioredoxin reductase (TR) 2-chloro-2,4-nitrobenzene and 2,6-dichloroindophenol. The competitive inhibitor TR inhibitor azelaic acid and the cytochrome P-450 inhibitor metyrapone lack any effects. The rates of reduction to the hydroxylamines and secondary amines were dependent on the lipid solubility of the nitroxides. Therefore, it can be assumed that the nitroxides must enter the cells for their bioreduction. The mostly discussed intracellular nitroxide reducing substances ascorbic acid and glutathione were unable to form the secondary amines. In conclusion, our results suggest that the secondary amine represents one of the major metabolites of nitroxides besides the hydroxylamine inside keratinocytes formed via the flavoenzyme thioredoxin reductase most probably. Further metabolic conversions were detected with 4-oxo-2,2,6,6-tetramethylpiperidine-1-oxyl and the benzoate of 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl as substrates.
PMID:10232828 Kroll C et al; Free Radic Biol Med 26 (7-8): 850-7 (1999)
The antiproliferative effect of Tempol, a stable nitroxide free radical, was investigated on the p53-negative human leukemia cell line HL60. A concentration- and time-dependent inhibition of cell growth was observed that appears to be due to induction of apoptosis. Involvement of oxidative stress is indicated by a concentration-dependent increase in intracellular peroxides and a parallel decrease in total cellular glutathione; in addition, increased survival rates were observed in cells simultaneously treated with Tempol and the antioxidant N-acetylcysteine. Tempol did not affect the relative levels of Bax and Bcl2, whereas p21(WAF1/CIP1) was enhanced in a concentration- and time-dependent fashion; this effect was partially inhibited by N-acetylcysteine, was maintained for up to 8 hr after Tempol removal, and seemed to depend on continuing protein synthesis. The increase in p21(WAF1/CIP1) was accompanied by a parallel accumulation of cells in the G(1) phase of the cycle and by a decrease in the 110 kDa form of pRb. Our results suggest that p53-independent induction of p21(WAF1/CIP1) mediates the antiproliferative effect of Tempol; on the basis of this observation, the nitroxide could be proposed as an useful adjunct to the treatment of p53-deficient tumors, which are often refractory to standard chemotherapy.
PMID:11033415 Gariboldi MB et al; Free Radic Biol Med. 2000 Oct 1;29(7):633-41 (2000)
A variety of mechanisms has been suggested for cocaine toxicity, including the possibility that cocaine induces an increase in oxidative stress (OS) due to excessive oxidation of dopamine (e.g. dopamine quinine), or by redox cycling of cocaine oxidized metabolites. However, the association between oxidative status in the brain and cocaine induced-behavior is poorly understood. Therefore, we examined the ability of the unique antioxidant tempol to attenuate cocaine-induced oxidative damage and behavioral response. Acute cocaine treatment significantly elevated OS markers in prefrontal cortex (PFC) and nucleus accumbens (NAc) in rats, both in slices and following a single cocaine injection, which corresponded with a decrease in total antioxidant capacity (TAC). Tempol, at the optimal concentration we determined that was needed to observe an antioxidant non-toxic effect in vitro (1 mM) and in vivo (200 mg/kg), completely abolished the elevation of OS markers and prevented the reduction in TAC in these areas. Importantly, tempol injections, at a dose that does not affect the basal levels of locomotor activity, attenuated both the development and expression of cocaine-induced locomotor sensitization. Finally, in cocaine-sensitized animals, tempol prevented the elevation of OS markers in both PFC and NAc. Our findings suggest that oxidation of specific sites in the brain reward system by cocaine is accompanied with behavioral changes. Tempol has a neuro-protective effect against cocaine toxicity in these regions, and it may be beneficial in the treatment of cocaine addiction.
PMID:18619523 Numa R et al; Neuroscience 155 (3): 649-58 (2008)
TEMPOL (4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl) is a stable nitroxyl antioxidant. Previous studies have suggested that TEMPOL is protective in acute disorders thought to involve reactive oxygen species (ROS), such as ischemic stroke and cardiac reperfusion injury. Oxidized TEMPOL can be recycled to its redox-active reducing form by co-administration with polynitroxylated albumin, making it a candidate as a pharmacological "reservoir" for reducing potential of use in chronic disorders involving ROS. The present studies examine the efficacy of TEMPOL in cell culture and animal models of the central and peripheral dysfunction associated with Parkinson's disease, a disorder in the pathogenesis of which ROS generated from dopamine have been implicated. Antioxidants have been proposed as both preventive and symptomatic therapy for Parkinson's disease. TEMPOL protects MN9D dopaminergic mesencephalic cells in culture from 6-hydroxydopamine (6-OHDA)-induced apoptosis. Translocation of the p65 component of NF-kappaB to the nucleus accompanies protection by TEMPOL. In vivo, intraperitoneal TEMPOL protects mice from intrastriatal 6-OHDA-induced cell and dopamine metabolite loss in the striatum. TEMPOL also protects mice against the 6-OHDA-induced rotational behavior elicited by intrastriatal administration of d-amphetamine. In addition, TEMPOL protects mice from the ptosis, activity level decrement, and mortality induced by intraperitoneal administration of 6-OHDA, a model of autonomic dysfunction in Parkinson's disease. Adjunctive use of polynitroxylated albumin enhances the in vitro and in vivo effects of TEMPOL.
PMID:16144694 Liang Q et al; Biochem Pharmacol 70 (9): 1371-81 (2005)
Reactive oxygen species (ROS) generated from dopamine and its oxidation products have been implicated in the pathogenesis and toxicity from treatment of Parkinson's disease-associated autonomic neuropathy, and antioxidant therapies have been proposed as treatment and prophylaxis for this disorder. However, many antioxidants are rapidly and, under physiological conditions, irreversibly oxidized, rendering them redox-inactive. We have examined the potential of 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl and polynitroxylated albumin (TEMPOL/PNA), an antioxidant complex that facilitates recycling of inactivated antioxidant to its redox-active form, as a protective agent against the toxicity of the catecholaminergic ROS generator, 6-hydroxydopamine (6-OHDA). TEMPOL/PNA is more effective against depression of activity level by 6-OHDA than the non-recycling antioxidant, TEMPOL, in a murine model of catecholaminergic oxidative damage. TEMPOL/PNA is also less toxic than TEMPOL in mice, allowing administration of higher doses of antioxidant. Both TEMPOL and TEMPOL/PNA give rise to prevention of apoptosis and to translocation of NF-kappaB from the cytoplasm to the nucleus of PC12 cells treated with 6-OHDA, but in vivo, TEMPOL/PNA maintains redox-active blood levels of TEMPOL for almost 5 hr, whereas administration of TEMPOL alone results in clearance of blood redox activity within 1 hr. PNA enhances the therapeutic index of TEMPOL, and the recycling antioxidant that results from their adjunctive administration may prove useful in disorders involving oxidative stress.
PMID:15158156 Weinberg A et al; Brain Res 1012 (1-2): 13-21 (2004)
For more Mechanism of Action (Complete) data for 1-Piperidinyloxy, 4-hydroxy-2,2,6,6-tetramethyl (26 total), please visit the HSDB record page.