

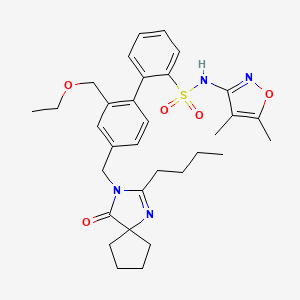

1. (1,1'-biphenyl)-2-sulfonamide, 4'-((2-butyl-4-oxo-1,3-diazaspiro(4.4)non-1-en-3-yl)methyl)-n-(4,5-dimethyl-3-isoxazolyl)-2'-(ethoxymethyl)-
2. 4'-((2-butyl-4-oxo-1,3-diazaspiro(4.4)non-1-en-3-yl)methyl)-n-(4,5-dimethyl-1,2-oxazol-3-yl)-2'-(ethoxymethyl)-2-biphenylsulfonamide
3. 4'-((2-butyl-4-oxo-1,3-diazaspiro(4.4)non-1-en-3-yl)methyl)-n-(4,5-dimethyl-1,2-oxazol-3-yl)-2'-(ethoxymethyl)biphenyl-2-sulfonamide
4. Re-021
1. 254740-64-2
2. Sparsentan (re-021)
3. Re-021
4. Sparsentan [usan]
5. Ps433540
6. Ps-433540
7. 9242ro5urm
8. Chembl539423
9. 2-[4-[(2-butyl-4-oxo-1,3-diazaspiro[4.4]non-1-en-3-yl)methyl]-2-(ethoxymethyl)phenyl]-n-(4,5-dimethyl-1,2-oxazol-3-yl)benzenesulfonamide
10. 4'-((2-butyl-4-oxo-1,3-diazaspiro[4.4]non-1-en-3-yl)methyl)-n-(4,5-dimethylisoxazol-3-yl)-2'-(ethoxymethyl)-[1,1'-biphenyl]-2-sulfonamide
11. Retrophin
12. (1,1'-biphenyl)-2-sulfonamide, 4'-((2-butyl-4-oxo-1,3-diazaspiro(4.4)non-1-en-3-yl)methyl)-n-(4,5-dimethyl-3-isoxazolyl)-2'-(ethoxymethyl)-
13. [1,1'-biphenyl]-2-sulfonamide, 4'-[(2-butyl-4-oxo-1,3-diazaspiro[4.4]non-1-en-3-yl)methyl]-n-(4,5-dimethyl-3-isoxazolyl)-2'-(ethoxymethyl)-
14. Unii-9242ro5urm
15. Dara
16. Compound 7 [pmid 15634011]
17. Dara-a
18. Ps 33540
19. Sparsentan [inn]
20. Sparsentan (usan/inn)
21. Sparsentan(ps433540)
22. Sparsentan [who-dd]
23. Schembl535109
24. Gtpl8448
25. Amy38147
26. Bcp23969
27. Ex-a3048
28. Bdbm50175523
29. S6665
30. Cs-7947
31. Db12548
32. Sb16876
33. Dara-a (dual Acting Receptor Antagonist Of Angiotension And Endothelin Receptors)
34. Ps-433540;re-021;dara
35. Ac-35179
36. Hy-17621
37. Sparsentan (ps-433540,re-021,dara)
38. D11776
39. F77045
40. A858308
41. L023324
42. Q27088845
43. 4''''-(2-butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-ylmethyl)-2''''-ethoxymethyl-biphenyl-2-sulfonic Acid (4,5-dimethyl-isoxazol-3-yl)-amide
44. 4''-(2-butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-ylmethyl)-2''-ethoxymethyl-biphenyl-2-sulfonic Acid (4,5-dimethyl-isoxazol-3-yl)-amide
45. 4'-((2-butyl-4-oxo-1,3-diazaspiro(4.4)non-1-en-3-yl)methyi)-n-(4,5- Dimethylisoxazol-3-yl)-2'-(ethoxymethyl)biphenyl-2-sulfonamide
46. 4'-((2-butyl-4-oxo-1,3-diazaspiro(4.4)non-1-en-3-yl)methyl)-n-(4,5-dimethyl-1,2-oxazol-3-yl)-2'-(ethoxymethyl)-2-biphenylsulfonamide
47. 4'-((2-butyl-4-oxo-1,3-diazaspiro(4.4)non-1-en-3-yl)methyl)-n-(4,5-dimethyl-1,2-oxazol-3-yl)-2'-(ethoxymethyl)biphenyl-2-sulfonamide
| Molecular Weight | 592.8 g/mol |
|---|---|
| Molecular Formula | C32H40N4O5S |
| XLogP3 | 5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 12 |
| Exact Mass | 592.27194156 g/mol |
| Monoisotopic Mass | 592.27194156 g/mol |
| Topological Polar Surface Area | 123 Ų |
| Heavy Atom Count | 42 |
| Formal Charge | 0 |
| Complexity | 1060 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Treatment of focal segmental glomerulosclerosis
Treatment of primary IgA nephropathy
Sparsentan, which possesses two clinically validated mechanisms of action in a single molecule, works by selectively blocking the action of two potent vasoconstrictor and mitogenic agents, angiotensin II (AII) and endothelin 1 (ET1), at their respective receptors. Sparsentan is highly selective (10,000-fold) for the AII receptor sub-type 1 and the ET receptor sub-type A. As such, Sparsentan combines the properties of an angiotensin receptor blocker (ARB) and an endothelin receptor antagonist (ERA) in the same molecule.

