

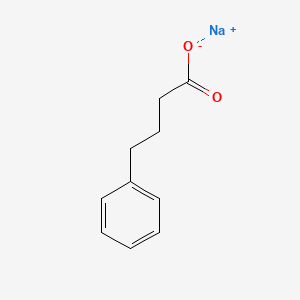

1. 4-phenylbutyrate
2. 4-phenylbutyric Acid
3. 4-phenylbutyric Acid, Calcium Salt
4. 4-phenylbutyric Acid, Sodium Salt
5. Ammonaps
6. Buphenyl
7. Sodium 4-phenylbutanoate
8. Sodium 4-phenylbutyrate
1. Sodium 4-phenylbutyrate
2. 1716-12-7
3. Sodium 4-phenylbutanoate
4. Buphenyl
5. Tributyrate
6. Ammonaps
7. Benzenebutanoic Acid, Sodium Salt
8. 4-phenylbutyric Acid Sodium Salt
9. Sodium;4-phenylbutanoate
10. 4-phenylbutyric Acid Sodium
11. 4-phenylbutyric Acid, Sodium Salt
12. Acer-001
13. Chebi:75316
14. Nt6k61736t
15. Mfcd00800247
16. Nsc-657802
17. Amx0035 Component Sodium Phenylbutyrate
18. Dsstox_cid_20948
19. Dsstox_rid_79606
20. Dsstox_gsid_40948
21. Pheburane
22. 4pba
23. Sodium Phenylbutyrate [usan]
24. Cas-1716-12-7
25. Ncgc00018113-03
26. Nsc 657802
27. Ammonapse
28. Lunaphen
29. Unii-nt6k61736t
30. Napb
31. Phenylbutyrate Na
32. Sodium Phenylbutyrate [usan:ban]
33. Buphenyl (tn)
34. El-532
35. Vp-101
36. 4-phenylbutyric Acid Sodium 100 Microg/ml In Acetonitrile:water
37. Sodium-4-phenylbutyrate
38. Chembl1746
39. Sodium 4-phenylbutyrate-d11
40. Phenylbutyric Acid Sodium Salt
41. Schembl125792
42. 4-pb
43. Sodium 4-phenylbutyrate, 95%
44. Dtxsid7040948
45. Cmk-304
46. Sodium Phenylbutyrate (jan/usp)
47. Hms2089b22
48. Hms3269n07
49. Hms3413b10
50. Hms3652k18
51. Hms3677b10
52. Hms3885p05
53. Sodium Phenylbutyrate [mi]
54. Sodium Phenylbutyrate [jan]
55. Amy13389
56. Sodium 4-phenylbutyrate;tributyrate
57. Tox21_110826
58. Phenylbutyrate Sodium [vandf]
59. S4125
60. Sodium Phenylbutyrate [mart.]
61. Akos006344763
62. Akos015967141
63. Sodium Phenylbutyrate [usp-rs]
64. Sodium Phenylbutyrate [who-dd]
65. Tox21_110826_1
66. Ccg-213730
67. Ccg-265014
68. Cs-1397
69. Sodium Phenylbutyrate [ema Epar]
70. Sodium Phenylbutyrate, >=98% (hplc)
71. Ncgc00018113-07
72. Ncgc00167830-01
73. As-13914
74. Hy-15654
75. Sy067225
76. Sodium Phenylbutyrate [orange Book]
77. Sodium Phenylbutyrate [ep Monograph]
78. A4107
79. Ft-0619402
80. O0511
81. Sodium Phenylbutyrate [usp Monograph]
82. Sw219199-1
83. D05868
84. F14969
85. Amx-0035 Component Sodium Phenylbutyrate
86. A811337
87. J-010746
88. J-524277
89. Q7553358
90. Sodium Phenylbutyrate, European Pharmacopoeia (ep) Reference Standard
91. Sodium Phenylbutyrate, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 186.18 g/mol |
|---|---|
| Molecular Formula | C10H11NaO2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 4 |
| Exact Mass | 186.06567387 g/mol |
| Monoisotopic Mass | 186.06567387 g/mol |
| Topological Polar Surface Area | 40.1 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 142 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 4 | |
|---|---|
| Drug Name | Buphenyl |
| PubMed Health | Sodium Phenylbutyrate (By mouth) |
| Drug Classes | Hyperammonemia Agent, Nutritive Agent |
| Drug Label | BUPHENYL (sodium phenylbutyrate) Tablets for oral administration and BUPHENYL (sodium phenylbutyrate) Powder for oral, nasogastric, or gastrostomy tube administration contain sodium phenylbutyrate. Sodium phenylbutyrate is an off-white crystalline su... |
| Active Ingredient | Sodium phenylbutyrate |
| Dosage Form | Tablet; Powder |
| Route | Oral |
| Strength | 3gm/teaspoonful; 500mg |
| Market Status | Prescription |
| Company | Hyperion Theraps |
| 2 of 4 | |
|---|---|
| Drug Name | Sodium phenylbutyrate |
| PubMed Health | Sodium Phenylbutyrate (By mouth) |
| Drug Classes | Hyperammonemia Agent, Nutritive Agent |
| Drug Label | BUPHENYL (sodium phenylbutyrate) Tablets for oral administration and BUPHENYL (sodium phenylbutyrate) Powder for oral, nasogastric, or gastrostomy tube administration contain sodium phenylbutyrate. Sodium phenylbutyrate is an off-white crystalline su... |
| Active Ingredient | Sodium phenylbutyrate |
| Dosage Form | Tablet; Powder |
| Route | Oral |
| Strength | 500mg; 3gm/teaspoonful |
| Market Status | Prescription |
| Company | Sigmapharm Labs; Ampolgen |
| 3 of 4 | |
|---|---|
| Drug Name | Buphenyl |
| PubMed Health | Sodium Phenylbutyrate (By mouth) |
| Drug Classes | Hyperammonemia Agent, Nutritive Agent |
| Drug Label | BUPHENYL (sodium phenylbutyrate) Tablets for oral administration and BUPHENYL (sodium phenylbutyrate) Powder for oral, nasogastric, or gastrostomy tube administration contain sodium phenylbutyrate. Sodium phenylbutyrate is an off-white crystalline su... |
| Active Ingredient | Sodium phenylbutyrate |
| Dosage Form | Tablet; Powder |
| Route | Oral |
| Strength | 3gm/teaspoonful; 500mg |
| Market Status | Prescription |
| Company | Hyperion Theraps |
| 4 of 4 | |
|---|---|
| Drug Name | Sodium phenylbutyrate |
| PubMed Health | Sodium Phenylbutyrate (By mouth) |
| Drug Classes | Hyperammonemia Agent, Nutritive Agent |
| Drug Label | BUPHENYL (sodium phenylbutyrate) Tablets for oral administration and BUPHENYL (sodium phenylbutyrate) Powder for oral, nasogastric, or gastrostomy tube administration contain sodium phenylbutyrate. Sodium phenylbutyrate is an off-white crystalline su... |
| Active Ingredient | Sodium phenylbutyrate |
| Dosage Form | Tablet; Powder |
| Route | Oral |
| Strength | 500mg; 3gm/teaspoonful |
| Market Status | Prescription |
| Company | Sigmapharm Labs; Ampolgen |
Treatment of chronic management of urea-cycle disorders.
Ammonaps is indicated as adjunctive therapy in the chronic management of urea cycle disorders, involving deficiencies of carbamylphosphate synthetase, ornithine transcarbamylase or
argininosuccinate synthetase.
It is indicated in all patients with neonatal-onset presentation (complete enzyme deficiencies, presenting within the first 28 days of life). It is also indicated in patients with late-onset disease
(partial enzyme deficiencies, presenting after the first month of life) who have a history of hyperammonaemic encephalopathy.
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
A16AX03
A16AX03
A - Alimentary tract and metabolism
A16 - Other alimentary tract and metabolism products
A16A - Other alimentary tract and metabolism products
A16AX - Various alimentary tract and metabolism products
A16AX03 - Sodium phenylbutyrate

