
1. Ahr 438
2. Skelaxin
1. 1665-48-1
2. Skelaxin
3. Zorane
4. Methaxalonum
5. Metaxalon
6. Methoxolone
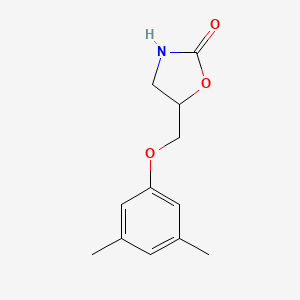
7. 5-((3,5-dimethylphenoxy)methyl)oxazolidin-2-one
8. Metazalone
9. Metazolone
10. Metaxolone
11. Ahr-438
12. Metaxalonum [latin]
13. Metassalone [dcit]
14. 5-[(3,5-dimethylphenoxy)methyl]-1,3-oxazolidin-2-one
15. 2-oxazolidinone, 5-[(3,5-dimethylphenoxy)methyl]-
16. Ahr 438
17. Metaxalonum [inn-latin]
18. Metaxalona [inn-spanish]
19. Nsc 170959
20. 5-(3,5-xyloloxymethyl)oxazolidin-2-one
21. Cl 39,148
22. Flexura
23. 5-((3,5-xylyloxy)methyl)-2-oxazolidinone
24. 5-[(3,5-xylyloxy)methyl]-2-oxazolidinone
25. 2-oxazolidinone, 5-((3,5-xylyloxy)methyl)-
26. 5-((3,5-dimethylphenoxy)methyl)-2-oxazolidinone
27. Mfcd00867700
28. Nsc170959
29. 5-[(3,5-dimethylphenoxy)methyl]oxazolidin-2-one
30. Nsc-170959
31. 2-oxazolidinone, 5-((3,5-dimethylphenoxy)methyl)-
32. 5-(3,5-dimethylphenoxymethyl)-1,3-oxazolidin-2-one
33. Mls003106749
34. 1nma9j598y
35. Chebi:6797
36. 5-[(3,5-dimethylphenoxy)methyl]-2-oxazolidinone
37. Ncgc00095116-01
38. Dsstox_cid_3269
39. 2-oxazolidinone, 5-[(3,5-xylyloxy)methyl]-
40. Dsstox_rid_76949
41. Dsstox_gsid_23269
42. Metaxalona [spanish]
43. Metassalone
44. Metaxalona
45. Metaxalonum
46. Cas-1665-48-1
47. Metaxalone [usan:inn:ban]
48. Hsdb 3236
49. Sr-05000001978
50. Einecs 216-777-6
51. 5-((3,5-dimethylphenoxy)methyl)-1,3-oxazolidin-2-one
52. Brn 0884592
53. Unii-1nma9j598y
54. .meta.xalone
55. .meta.zalone
56. .meta.zolone
57. .meta.xalon
58. Metaxalone Solution
59. Skelaxin (tn)
60. Spectrum_001741
61. Metaxalone [mi]
62. Specplus_000656
63. Metaxalone (usp/inn)
64. Metaxalone [inn]
65. Spectrum2_000548
66. Spectrum3_001666
67. Spectrum4_000612
68. Spectrum5_001685
69. Metaxalone [hsdb]
70. Metaxalone [usan]
71. Metaxalone [vandf]
72. 5-(3,5-dimethylphenoxymethyl)-2-oxazolidinone
73. Metaxalone [mart.]
74. Metaxalone [usp-rs]
75. Metaxalone [who-dd]
76. Oprea1_438855
77. Schembl34908
78. Bspbio_003451
79. Kbiogr_001164
80. Kbioss_002221
81. Divk1c_006752
82. Spectrum1504229
83. Spbio_000595
84. Ahr438
85. Gtpl7609
86. Chembl1079604
87. Dtxsid3023269
88. Metaxalone [orange Book]
89. Metaxalone, >=98% (hplc)
90. Kbio1_001696
91. Kbio2_002221
92. Kbio2_004789
93. Kbio2_007357
94. Kbio3_002671
95. Hms1922h07
96. Hms2093c22
97. Metaxalone [usp Monograph]
98. Pharmakon1600-01504229
99. Bcp28377
100. Hy-b0678
101. Tox21_111428
102. Wln: T5mvotj D1or C1 E1
103. 2-oxazolidinone,5-xylyloxy)methyl]-
104. Ccg-39592
105. Nsc758703
106. S3730
107. Stl450994
108. Stl451511
109. Akos009035315
110. Tox21_111428_1
111. Db00660
112. Fs-3218
113. Nsc-758703
114. Ncgc00095116-02
115. Ncgc00095116-03
116. Ncgc00095116-05
117. Smr001821638
118. Sy052772
119. Sbi-0052859.p002
120. 2-oxazolidinone,5-dimethylphenoxy)methyl]-
121. Db-043656
122. Am20060525
123. Ft-0603568
124. M2578
125. 5-(3,5-dimethylphenoxy)methyl-2-oxazolidinone
126. C07934
127. D00773
128. Ab00053284_04
129. 665m481
130. A810747
131. J-010295
132. Q6823309
133. Sr-05000001978-1
134. Sr-05000001978-3
135. Brd-a94709349-001-02-6
136. Brd-a94709349-001-03-4
137. 5-[(3,5-dimethylphenoxy)methyl]-1,3-oxazolidin-2-one #
138. 5-{[(3,5-dimethylphenyl)oxy]methyl}-1,3-oxazolidin-2-one
139. 5-[(3,5-dimethylphenoxy)methyl]-4,5-dihydro-1,3-oxazol-2-ol
140. Metaxalone, United States Pharmacopeia (usp) Reference Standard
141. Metaxalone Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 221.25 g/mol |
|---|---|
| Molecular Formula | C12H15NO3 |
| XLogP3 | 2.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 221.10519334 g/mol |
| Monoisotopic Mass | 221.10519334 g/mol |
| Topological Polar Surface Area | 47.6 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 247 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Metaxalone |
| PubMed Health | Metaxalone (By mouth) |
| Drug Classes | Skeletal Muscle Relaxant, Centrally Acting |
| Drug Label | DESCRIPTION Metaxalone tablets are available as an 800 mg oval, convex pink tablet.Chemically, metaxalone is 5-[(3,5- dimethylphenoxy) methyl]-2-oxazolidinone. The empirical formula is C12H15NO3, which corresponds to a molecular weight of 221.25. The... |
| Active Ingredient | Metaxalone |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 400mg; 800mg |
| Market Status | Tentative Approval; Prescription |
| Company | Corepharma; Amneal Pharms; Sandoz |
| 2 of 4 | |
|---|---|
| Drug Name | Skelaxin |
| PubMed Health | Metaxalone (By mouth) |
| Drug Classes | Skeletal Muscle Relaxant, Centrally Acting |
| Drug Label | SKELAXIN (metaxalone) is available as an 800 mg oval, scored pink tablet.Chemically, metaxalone is 5-[(3,5- dimethylphenoxy) methyl]-2-oxazolidinone. The empirical formula is C12H15NO3, which corresponds to a molecular weight of 221.25. The structu... |
| Active Ingredient | Metaxalone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 800mg |
| Market Status | Prescription |
| Company | King Pharms |
| 3 of 4 | |
|---|---|
| Drug Name | Metaxalone |
| PubMed Health | Metaxalone (By mouth) |
| Drug Classes | Skeletal Muscle Relaxant, Centrally Acting |
| Drug Label | DESCRIPTION Metaxalone tablets are available as an 800 mg oval, convex pink tablet.Chemically, metaxalone is 5-[(3,5- dimethylphenoxy) methyl]-2-oxazolidinone. The empirical formula is C12H15NO3, which corresponds to a molecular weight of 221.25. The... |
| Active Ingredient | Metaxalone |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 400mg; 800mg |
| Market Status | Tentative Approval; Prescription |
| Company | Corepharma; Amneal Pharms; Sandoz |
| 4 of 4 | |
|---|---|
| Drug Name | Skelaxin |
| PubMed Health | Metaxalone (By mouth) |
| Drug Classes | Skeletal Muscle Relaxant, Centrally Acting |
| Drug Label | SKELAXIN (metaxalone) is available as an 800 mg oval, scored pink tablet.Chemically, metaxalone is 5-[(3,5- dimethylphenoxy) methyl]-2-oxazolidinone. The empirical formula is C12H15NO3, which corresponds to a molecular weight of 221.25. The structu... |
| Active Ingredient | Metaxalone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 800mg |
| Market Status | Prescription |
| Company | King Pharms |
Metaxalone is used as an adjunct to rest, physical therapy, analgesics, and other measures for the relief of discomfort associated with acute, painful musculoskeletal conditions. /Included in US product label/
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 1394
The most frequent adverse effects of metaxalone are drowsiness, dizziness, headache, nervousness or irritability, nausea, vomiting, and GI upset. Other adverse effects include confusion, anorexia, dry mouth, and urinary retention. Exacerbation of tonic-clonic (grand mal) seizures has also been reported.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 1394
Hypersensitivity reactions and rash (with or without pruritus) have occurred in patients receiving metaxalone. Anaphylactoid reactions, leukopenia, hemolytic anemia, and jaundice have occurred rarely. Abnormalities in liver function tests, such as increased serum concentrations of AST (SGOT), ALT (SGPT), alkaline phosphatase, and bilirubin, and increased sulfobromophthalein (BSP) retention and thymol turbidity, have occurred in patients receiving metaxalone. Although a causal relationship to metaxalone has not been established, nephrotoxicity and proteinuria have occurred rarely during treatment with the drug; pyuria and nephrolithiasis have also been reported.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 1394
Safety and efficacy of metaxalone in children 12 years of age or younger have not been established; therefore, the drug should not be administered to children in this age group.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 1394
Patients should be warned that metaxalone may impair ability to perform hazardous activities requiring mental alertness or physical coordination, such as operating machinery or driving a motor vehicle. In addition, patients should be warned that additive CNS depression may occur when the drug is administered concomitantly with other CNS depressants, including alcohol. Metaxalone should be used with caution in geriatric patients and in patients with hepatic or renal impairment. Liver function studies should be performed periodically during metaxalone therapy in patients with preexisting liver damage. The drug is contraindicated in patients with substantially impaired hepatic or renal function, known hypersensitivity to the drug or any ingredient in the formulation, or a history of drug-induced, hemolytic, or other anemias.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 1394
For more Drug Warnings (Complete) data for METAXALONE (8 total), please visit the HSDB record page.
For the treatment of painful peripheral musculoskeletal conditions and spasticity from upper motor neuron syndromes.
FDA Label
Metaxalone is a skeletal muscle relaxant indicated as an adjunct to rest, physical therapy, and other measures for the relief of discomforts associated with acute, painful musculoskeletal conditions. The mode of action of this drug has not been clearly identified, but may be related to its sedative properties. Metaxalone does not directly relax tense skeletal muscles in man.
Neuromuscular Agents
Drugs used for their actions on skeletal muscle. Included are agents that act directly on skeletal muscle, those that alter neuromuscular transmission (NEUROMUSCULAR BLOCKING AGENTS), and drugs that act centrally as skeletal muscle relaxants (MUSCLE RELAXANTS, CENTRAL). Drugs used in the treatment of movement disorders are ANTI-DYSKINESIA AGENTS. (See all compounds classified as Neuromuscular Agents.)
Absorption
The absolute bioavailability of metaxalone from Skelaxin tablets is not known.
Route of Elimination
Metaxalone is metabolized by the liver and excreted in the urine as unidentified metabolites.
Volume of Distribution
800 L
Clearance
68 +/- 50 L/h [Subjects received 1400mg tablet under fasted conditions]
66 +/- 51 L/h [Subjects received 2400 mg tablets under fasted conditions]
Although plasma protein binding and absolute bioavailability of metaxalone are not known, the apparent volume of distribution (V/F ~ 800 L) and lipophilicity (log P = 2.42) of metaxalone suggest that the drug is extensively distributed in the tissues. Metaxalone is metabolized by the liver and excreted in the urine as unidentified metabolites.
US Natl Inst Health; DailyMed. Current Medication Information for Skelaxin (metaxalone) tablet (June 2007). Available from, as of October 15, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=4505
Peak plasma concentrations of metaxalone occur approximately 3 hours after a 400 mg oral dose under fasted conditions. Thereafter, metaxalone concentrations decline log-linearly with a terminal half-life of 9.0 + or - 4.8 hours. Doubling the dose of skelaxin from 400 mg to 800 mg results in a roughly proportional increase in metaxalone exposure as indicated by peak plasma concentrations (Cmax) and area under the curve (AUC). Dose proportionality at doses above 800 mg has not been studied. The absolute bioavailability of metaxalone is not known
US Natl Inst Health; DailyMed. Current Medication Information for Skelaxin (metaxalone) tablet (June 2007). Available from, as of October 15, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=4505
Probably hepatic.
Biotransformations...studied in dogs and in man. 5-(3-methyl-5-carboxyphenoxymethyl)-2-oxazolidinone, major metabolite, is excreted in urine and feces; this also occurs in urine as ester glucuronide. Fission of ether linkage...affords...3,5-xylenol and 5-hydroxymethyloxazolidinone. Oxazolidinone ring is stable in mammals.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 1: A Review of the Literature Published Between 1960 and 1969. London: The Chemical Society, 1970., p. 175
Yields 5-(5-carboxy-3-methylphenoxymethyl)-2-oxazolidone in man & in dogs, yields 3,5-dimethylphenol in man and in dogs. /From table/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. D-93
Metaxalone is metabolized by the liver and excreted in the urine as unidentified metabolites.
US Natl Inst Health; DailyMed. Current Medication Information for Skelaxin (metaxalone) tablet (June 2007). Available from, as of October 15, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=4505
9.2 (+/- 4.8) hours
Terminal half-life /is/ 9.0 + or - 4.8 hours
US Natl Inst Health; DailyMed. Current Medication Information for Skelaxin (metaxalone) tablet (June 2007). Available from, as of October 15, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=4505
The mechanism of action of metaxalone in humans has not been established, but may be due to general central nervous system depression.
Metaxalone is a CNS depressant that has sedative and skeletal muscle relaxant effects. The precise mechanism of action of the drug is not known. The skeletal muscle relaxant effects of orally administered metaxalone are minimal and are probably related to its sedative effect. The drug does not directly relax skeletal muscle and, unlike neuromuscular blocking agents, does not depress neuronal conduction, neuromuscular transmission, or muscle excitability.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 1395
The mechanism of action of metaxalone in humans has not been established, but may be due to general central nervous system depression. Metaxalone has no direct action on the contractile mechanism of striated muscle, the motor end plate or the nerve fiber.
US Natl Inst Health; DailyMed. Current Medication Information for Skelaxin (metaxalone) tablet (June 2007). Available from, as of October 15, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=4505