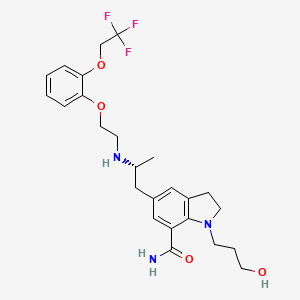

1. 1-(3-hydroxypropyl)-5-(2-(2-(2-(2,2,2-trifluoroethoxy)phenoxy)ethylamino)propyl)indoline-7-carboxamide
2. Kmd 3213
3. Kmd-3213
4. Rapaflo
1. 160970-54-7
2. Rapaflo
3. Urief
4. Silodyx
5. Urorec
6. Kmd-3213
7. Kmd 3213
8. Rapilif
9. Silodal
10. Kad 3213
11. Kad-3213
12. Kso-0400
13. Cuz39luy82
14. Chembl24778
15. 1-(3-hydroxypropyl)-5-[(2r)-2-[2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethylamino]propyl]-2,3-dihydroindole-7-carboxamide
16. 2,3-dihydro-1-(3-hydroxypropyl)-5-[(2r)-2-[[2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethyl]amino]propyl]-1h-indole-7-carboxamide
17. (-)-1-(3-hydroxypropyl)-5-[(2r)-2-[[2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethyl]amino]propyl]-2,3-di-hydro-1h-indole-7-carboxamide
18. Rapflo
19. Silodosin [inn]
20. ( C)-1-(3-hydroxypropyl)-5-[(2r)-2-[[2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethyl]amino]propyl]-2,3-di-hydro-1h-indole-7-carboxamide
21. (-)-1-(3-hydroxypropyl)-5-((2r)-2-((2-(2-(2,2,2-trifluoroethoxy)phenoxy)ethyl)amino)propyl)-2,3-dihydro-1h-indole-7-carboxamide
22. 1-(3-hydroxypropyl)-5-[(2r)-2-({2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethyl}amino)propyl]-2,3-dihydro-1h-indole-7-carboxamide
23. 1h-indole-7-carboxamide, 2,3-dihydro-1-(3-hydroxypropyl)-5-[(2r)-2-[[2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethyl]amino]propyl]-
24. Silodosin [inn:ban]
25. Unii-cuz39luy82
26. Urief, Rapaflo
27. 2,3-dihydro-1-(3-hydroxypropyl)-5-((2r)-2-((2-(2-(2,2,2-trifluoroethoxy)phenoxy)ethyl)amino)propyl)-1h-indole-7-carboxamide
28. Rapaflo (tn)
29. Silodosin (rapaflo)
30. Urief (tn)
31. Silodosin (r-isomer)
32. Silodosin [jan]
33. Silodosin [mi]
34. Silodosin [vandf]
35. Silodosin (jp17/inn)
36. Silodosin [mart.]
37. Silodosin [who-dd]
38. Gtpl493
39. Silodosin [ema Epar]
40. Mls006010022
41. Schembl136973
42. Silodosin [orange Book]
43. Silodosin, >=98% (hplc)
44. Dtxsid40167045
45. Kmd3213
46. Chebi:135929
47. Hms3715b06
48. Hms3884o21
49. Bcp02143
50. Zinc3806063
51. Bdbm50160154
52. Mfcd00930170
53. S1613
54. Akos005145899
55. Kad 3213;kmd 3213
56. Bs-1011
57. Ccg-221202
58. Cs-0284
59. Db06207
60. Ncgc00345882-03
61. 1-(3-hydroxypropyl)-5-(2-(2-(2-(2,2,2-trifluoroethoxy)phenoxy)ethylamino)propyl)indoline-7-carboxamide
62. Ac-22605
63. Hy-10122
64. Smr004701206
65. Am20090780
66. I1128
67. Sw219765-1
68. D01965
69. 970s649
70. A810210
71. Q411770
72. Sr-01000944157
73. Q-102517
74. Sr-01000944157-1
75. (r)-1-(3-hydroxypropyl)-5-(2-(2-(2-(2,2,2-trifluoroethoxy)phenoxy)ethylamino)propyl)indoline-7-carboxamide
76. (r)-5-[2-[[2-[2-(2,2,2-trifluoro-ethoxy)phenoxy]ethyl]amino]propyl]-1-(3-hydroxypropyl)-2,3-dihydro-1h-indole-7-carboxamide
77. 1-(3-hydroxy-propyl)-5-((r)-2-{2-[2-(2,2,2-trifluoro-ethoxy)-phenoxy]-ethylamino}-propyl)-2,3-dihydro-1h-indole-7-carboxylic Acid Amide
78. 1-(3-hydroxypropyl)-5-[(2r)-2-({2-[2-(2,2,2-trifluoroethoxy)phenoxy]-ethyl}amino)propyl]-2,3-dihydro-1h-indole-7-carboxamide
79. 1-(3-hydroxypropyl)-5-[(2r)-2-[[2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethyl]amino]propyl]-2,3-dihydro-1h-indole-7-carboxamide; 1-(3-hydroxypropyl)-5-[(2r)-2-({2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethyl}amino)propyl]-2,3-dihydro-1h-indole-7-carboxamide; (-)-1
| Molecular Weight | 495.5 g/mol |
|---|---|
| Molecular Formula | C25H32F3N3O4 |
| XLogP3 | 3.6 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 13 |
| Exact Mass | 495.23449100 g/mol |
| Monoisotopic Mass | 495.23449100 g/mol |
| Topological Polar Surface Area | 97 Ų |
| Heavy Atom Count | 35 |
| Formal Charge | 0 |
| Complexity | 654 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Rapaflo |
| PubMed Health | Silodosin (By mouth) |
| Drug Classes | Benign Prostatic Hypertrophy Agent |
| Drug Label | RAPAFLO is the brand name for silodosin, a selective antagonist of alpha-1 adrenoreceptors. The chemical name of silodosin is 1-(3-Hydroxypropyl)-5-[(2R)-2-({2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethyl}amino)propyl]-2,3-dihydro-1H-indole-7-carboxamide... |
| Active Ingredient | Silodosin |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 8mg; 4mg |
| Market Status | Prescription |
| Company | Watson Labs |
| 2 of 2 | |
|---|---|
| Drug Name | Rapaflo |
| PubMed Health | Silodosin (By mouth) |
| Drug Classes | Benign Prostatic Hypertrophy Agent |
| Drug Label | RAPAFLO is the brand name for silodosin, a selective antagonist of alpha-1 adrenoreceptors. The chemical name of silodosin is 1-(3-Hydroxypropyl)-5-[(2R)-2-({2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethyl}amino)propyl]-2,3-dihydro-1H-indole-7-carboxamide... |
| Active Ingredient | Silodosin |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 8mg; 4mg |
| Market Status | Prescription |
| Company | Watson Labs |
Silodosin is indicated for the treatment of the signs and symptoms of benign prostatic hyperplasia (BPH). It is not indicated for the treatment of hypertension.
FDA Label
Treatment of the signs and symptoms of benign prostatic hyperplasia (BPH)
Treatment of the signs and symptoms of benign prostatic hyperplasia (BPH).
Treatment of the signs and symptoms of benign prostatic hyperplasia (BPH) in adult men.
Silodosin is an antagonist of 1-adrenoceptors. It has the highest selectivity for the 1A-adrenoceptor subtype, with a 162-fold greater affinity than 1B-adrenoceptor and about a 50-fold greater affinity than for 1D-adrenoceptor. In clinical trials, silodosin improved maximum urinary flow rate, voiding symptoms, and storage symptoms of benign prostatic hyperplasia. Following oral administration, silodosin had a rapid onset of effect in men, with early effects of relieving lower urinary tract symptoms occurring within two to six hours post-dose. Silodosin inhibited the human ether-a-go-go-related gene (HERG) tail current; however, it has weak cardiovascular effects. As with all 1-adrenoceptor antagonists blocking 1-adrenoceptors in the iris dilator muscle, silodosin may cause intraoperative floppy iris syndrome (IFIS), which is characterized by small pupils and iris billowing during cataract surgery in patients taking 1-AR antagonists.
Adrenergic alpha-1 Receptor Antagonists
Drugs that bind to and block the activation of ADRENERGIC ALPHA-1 RECEPTORS. (See all compounds classified as Adrenergic alpha-1 Receptor Antagonists.)
Urological Agents
Drugs used in the treatment of urological conditions and diseases such as URINARY INCONTINENCE and URINARY TRACT INFECTIONS. (See all compounds classified as Urological Agents.)
G04CA04
G04CA04
G04CA04
G04CA04
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
G - Genito urinary system and sex hormones
G04 - Urologicals
G04C - Drugs used in benign prostatic hypertrophy
G04CA - Alpha-adrenoreceptor antagonists
G04CA04 - Silodosin
Absorption
The absolute bioavailability is approximately 32%. Following oral administration of silodosin 8 mg once daily in healthy male subjects, Cmax was 61.6 27.54 ng/mL and AUC was 373.4 164.94 ng x hr/mL. The Tmax was 2.6 0.90 hours. Silodosin glucuronide or KMD-3213G, the main metabolite of silodosin, has an AUC three- or four fold higher than for the parent compound. A moderate fat or calorie meal reduces Cmax by 18% to 43% and AUC by 4% to 49%, as well as Tmax by about one hour. However, the US prescribing information recommends drug intake with meals to avoid the potential adverse effects associated with high plasma drug concentrations.
Route of Elimination
At 10 days following oral administration of radiolabelled silodosin, about 33.5% of the dose was recovered in urine and 54.9% was recovered in feces.
Volume of Distribution
Silodosin has an apparent volume of distribution of 49.5 L.
Clearance
After intravenous administration, the plasma clearance of silodosin was approximately 10 L/hour.
The main metabolite of silodosin is silodosin glucuronide (KMD-3213G), which is a pharmacologically active metabolite formed by direct glucuronide conjugation mediated by UDP-glucuronosyltransferase 2B7 (UGT2B7). Silodosin glucuronide reaches plasma exposure (AUC) approximately four times greater than that of silodosin. The second major metabolite, KMD-3293, is formed from dehydrogenation catalyzed by alcohol and aldehyde dehydrogenases. KMD-3293 has negligible pharmacological activity and reaches plasma exposures similar to that of silodosin. Silodosin is also metabolized by CYP3A4, which catalyzes the oxidation reaction. Other than glucuronidation, dehydrogenation, and oxidation as its main metabolic pathways, silodosin can also undergo dealkylation (KMD-3289), N-dealkylation, hydroxylation, glucosylation, and sulfate conjugation. Metabolites of silodosin can undergo a series of further metabolic pathways.
The elimination half-life of silodosin is 13.3 8.07 hours. KMD-3213G, the main metabolite of silodosin, has an extended half-life of approximately 24 hours.
The pathogenesis of benign prostatic hyperplasia is not fully understood: it is believed to involve several pathways, including inflammation, apoptosis, and cellular proliferation. Most drug therapies aim to alleviate symptoms of benign prostatic hyperplasia, silodosin included. Lower urinary tract symptoms of benign prostatic hyperplasia are categorized into three main groups: voiding or obstructive (hesitancy, slow stream, intermittency, incomplete emptying), storage or irritative (frequency, urgency, nocturia, urge urinary incontinence), and postmicturition (postvoid dribbling). Prostate contraction is the main contributor to lower urinary tract symptoms of benign prostatic hyperplasia. The smooth muscle tone of the prostate is regulated by 1A-adrenoceptors, which are the most highly expressed subtype of 1adrenoceptors in the human prostate tissue. It has been reported that blockade of 1A-adrenoceptors relieves bladder outlet obstruction. Blockade of 1D-adrenoceptors, another subtype found in prostate tissue, is believed to alleviate storage symptoms due to detrusor overactivity. 1-adrenoceptors are G protein-coupled receptors: upon binding of its natural ligand, norepinephrine and epinephrine, leads to the activation of phospholipase C and downstream signalling molecules, including inositol triphosphate and diacylglycerol. Ultimately, there is an increase in intracellular calcium levels and, consequently, smooth muscle contraction. Silodosin is an antagonist of 1-adrenoceptors, with the highest selectivity for the 1A-adrenoceptor subtype. By blocking the 1A-adrenoceptor signalling pathway, silodosin promotes prostatic and urethral smooth muscle relaxation, thereby improving lower urinary tract symptoms such as voiding. Silodosin also targets afferent nerves in the bladder, relieving bladder overactivity and storage symptoms.