

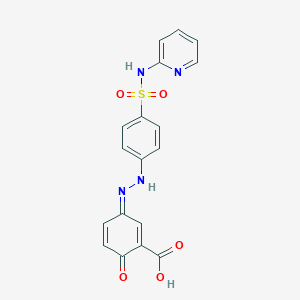

| Molecular Weight | 398.4 g/mol |
|---|---|
| Molecular Formula | C18H14N4O5S |
| XLogP3 | 2.3 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 6 |
| Exact Mass | 398.06849073 g/mol |
| Monoisotopic Mass | 398.06849073 g/mol |
| Topological Polar Surface Area | 146 A^2 |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 804 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Azulfidine |
| PubMed Health | Salicylate (Oral route, Rectal route) |
| Drug Classes | Antirheumatic, Gastrointestinal Agent |
| Drug Label | AZULFIDINE EN-tabs Tablets contain sulfasalazine, formulated in a delayed release tablet (enteric-coated), 500 mg, for oral administration.AZULFIDINE EN-tabs Tablets are film coated with cellulose acetate phthalate to retard disintegration of the tab... |
| Active Ingredient | Sulfasalazine |
| Dosage Form | Tablet; Suspension |
| Route | Oral |
| Strength | 500mg; 250mg/5ml |
| Market Status | Prescription |
| Company | Pharmacia And Upjohn |
| 2 of 6 | |
|---|---|
| Drug Name | Azulfidine en-tabs |
| PubMed Health | Sulfasalazine (By mouth) |
| Drug Label | AZULFIDINE EN-tabs Tablets contain sulfasalazine, formulated in a delayed release tablet (enteric-coated), 500 mg, for oral administration.AZULFIDINE EN-tabs Tablets are film coated with cellulose acetate phthalate to retard disintegration of the tab... |
| Active Ingredient | Sulfasalazine |
| Dosage Form | Tablet; Tablet, delayed release |
| Route | oral; Oral |
| Strength | 500mg |
| Market Status | Prescription |
| Company | Pharmacia Upjohn; Pharmacia And Upjohn |
| 3 of 6 | |
|---|---|
| Drug Name | Sulfasalazine |
| Drug Label | Sulfasalazine Tablets USP, 500 mg for Oral AdministrationTherapeutic classification: Anti-inflammatory agent.Chemical designation: 5-[[p-(2-Pyridylsulfamoyl)phenyl]azo]salicylic acid.Chemical Structure:The molecular weight of sulfasalazine is 398.39.... |
| Active Ingredient | Sulfasalazine |
| Dosage Form | Tablet; Tablet, delayed release |
| Route | Oral |
| Strength | 500mg |
| Market Status | Prescription |
| Company | Vintage Pharms; Watson Labs |
| 4 of 6 | |
|---|---|
| Drug Name | Azulfidine |
| PubMed Health | Salicylate (Oral route, Rectal route) |
| Drug Classes | Antirheumatic, Gastrointestinal Agent |
| Drug Label | AZULFIDINE EN-tabs Tablets contain sulfasalazine, formulated in a delayed release tablet (enteric-coated), 500 mg, for oral administration.AZULFIDINE EN-tabs Tablets are film coated with cellulose acetate phthalate to retard disintegration of the tab... |
| Active Ingredient | Sulfasalazine |
| Dosage Form | Tablet; Suspension |
| Route | Oral |
| Strength | 500mg; 250mg/5ml |
| Market Status | Prescription |
| Company | Pharmacia And Upjohn |
| 5 of 6 | |
|---|---|
| Drug Name | Azulfidine en-tabs |
| PubMed Health | Sulfasalazine (By mouth) |
| Drug Label | AZULFIDINE EN-tabs Tablets contain sulfasalazine, formulated in a delayed release tablet (enteric-coated), 500 mg, for oral administration.AZULFIDINE EN-tabs Tablets are film coated with cellulose acetate phthalate to retard disintegration of the tab... |
| Active Ingredient | Sulfasalazine |
| Dosage Form | Tablet; Tablet, delayed release |
| Route | oral; Oral |
| Strength | 500mg |
| Market Status | Prescription |
| Company | Pharmacia Upjohn; Pharmacia And Upjohn |
| 6 of 6 | |
|---|---|
| Drug Name | Sulfasalazine |
| Drug Label | Sulfasalazine Tablets USP, 500 mg for Oral AdministrationTherapeutic classification: Anti-inflammatory agent.Chemical designation: 5-[[p-(2-Pyridylsulfamoyl)phenyl]azo]salicylic acid.Chemical Structure:The molecular weight of sulfasalazine is 398.39.... |
| Active Ingredient | Sulfasalazine |
| Dosage Form | Tablet; Tablet, delayed release |
| Route | Oral |
| Strength | 500mg |
| Market Status | Prescription |
| Company | Vintage Pharms; Watson Labs |