

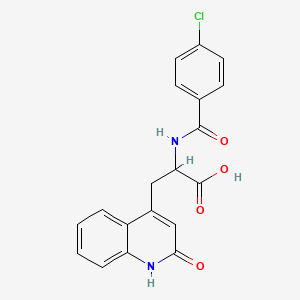

1. 2-(4-chlorobenzoylamino)-3-(2(1h)-quinolinon-4-yl)propionic Acid
2. Opc 12759
3. Opc-12759
4. Rebamipide, (+)-isomer
5. Rebamipide, (-)-isomer
1. 90098-04-7
2. Proamipide
3. Mucosta
4. 111911-87-6
5. Opc-12759
6. Rebamipide Hydrate
7. Pramipide
8. Rebator
9. 2-(4-chlorobenzoylamino)-3-(1,2-dihydro-2-oxo-4-quinolyl)propionic Acid
10. 139344-42-6
11. 2-[(4-chlorobenzoyl)amino]-3-(2-oxo-1h-quinolin-4-yl)propanoic Acid
12. Nsc-758955
13. 2-(4-chlorobenzamido)-3-(2-oxo-1,2-dihydroquinolin-4-yl)propanoic Acid
14. Lr583v32zr
15. Mfcd00866895
16. Ncgc00095161-01
17. 2-(4-chlorobenzamido)-3-[2(1h)-quinolinon-4-yl]propionic Acid
18. Dsstox_cid_25937
19. Dsstox_rid_81235
20. Dsstox_gsid_45937
21. Rebamipidum
22. Rebamipide [inn:jan]
23. 2-[(4-chlorobenzoyl)amino]-3-(2-hydroxyquinolin-4-yl)propanoic Acid
24. Rebamipidum [inn-latin]
25. 2-[[(4-chlorophenyl)-oxomethyl]amino]-3-(2-oxo-1h-quinolin-4-yl)propanoic Acid
26. Opc 12759
27. Cas-90098-04-7
28. Ccris 3585
29. Sr-05000001520
30. Unii-lr583v32zr
31. N-[(4-chlorophenyl)carbonyl]-3-(2-oxo-1,2-dihydroquinolin-4-yl)alanine
32. Dispersered72
33. 2-[(4-chlorophenyl)carbonylamino]-3-(2-oxidanylidene-1h-quinolin-4-yl)propanoic Acid
34. Rebamipide-[d4]
35. Mucosta (tn)
36. Rebamipide [mi]
37. Rebamipide [inn]
38. Rebamipide [jan]
39. Spectrum2_000039
40. Spectrum3_001959
41. Rebamipide (jp17/inn)
42. (+-)-2-(4-chlorobenzoylamino)-3-(2(1h)-quinolinon-4-yl)propionic Acid
43. (+-)-1,2-dihydro-alpha-((4-chlorobenzoyl)amino)-2-oxo-4-quinolinepropanoic Acid
44. Rebamipide [who-dd]
45. Bspbio_003559
46. Gtpl871
47. Mls006011883
48. Schembl221527
49. Spectrum1505310
50. Spbio_000137
51. Rebamipide 111911-87-6
52. Chembl1697771
53. Dtxsid8045937
54. Chebi:93814
55. Kbio3_002880
56. Opc-759
57. Hms1922b20
58. Hms2090l13
59. Hms3655l11
60. Hms3714a15
61. Pharmakon1600-01505310
62. Bcp07230
63. Hy-b0360
64. Opc12759
65. Tox21_111460
66. Bbl011328
67. Ccg-39619
68. Nsc758955
69. S2032
70. Stk577121
71. Stl146407
72. 2-(4-chlorobenzamido)-3-(2-oxo-1,2-dihydro-4-quinolyl)propanoic Acid
73. Akos005501649
74. Akos005721106
75. Tox21_111460_1
76. Ac-6841
77. Ac-7588
78. Db11656
79. Nsc 758955
80. 2-(4-chloro-benzoylamino)-3-(2-oxo-1,2-dihydro-quinolin-4-yl)-propionic Acid
81. 4-quinolinepropanoic Acid, 1,2-dihydro-alpha-((4-chlorobenzoyl)amino)-2-oxo-, (+-)-
82. Ncgc00095161-02
83. Ncgc00095161-03
84. Ncgc00095161-04
85. Ncgc00095161-05
86. Bc164330
87. Smr003309276
88. Sy057250
89. Vs-02924
90. Sbi-0207054.p001
91. Ft-0630971
92. Ft-0655225
93. R0085
94. Sw199113-2
95. D01121
96. Ab01275518-01
97. Ab01275518_02
98. Ab01275518_03
99. 911r876
100. A802443
101. A843443
102. A900081
103. Q-201660
104. Q7301602
105. Sr-05000001520-1
106. Sr-05000001520-2
107. Sr-05000001520-3
108. Brd-a15909516-001-02-5
109. Brd-a15909516-001-03-3
110. 2-(4-chlorobenzoylamino)-3-(2-quinolon-4-yl)propionic Acid
111. 2-(4-chlorobenzoylamino)-3-[2(1h)-quinolinon-4-yl] Propionic Acid
112. 2-(4-chlorobenzoylamino)-3-[2(1h)-quinolinon-4-yl]propionic Acid
113. N-[(4-chlorophenyl)carbonyl]-3-(2-hydroxyquinolin-4-yl)alanine
114. 2-(4-chlorobenzamido)-3-(2-oxo-1,2-dihydroquinolin-4-yl)propanoicacid
115. 2-(4-chlorobenzoylamino)-3-(2-oxo-1,2-dihydroquinolin-4-yl)propionic Acid
116. 4-quinolinepropanoicacid,a-[(4-chlorobenzoyl)amino]-1,2-dihydro-2-oxo-
117. (+/-)-.alpha.-(p-chlorobenzamido)-1,2-dihydro-2-oxo-4-quinolinepropionic Acid
118. (+/-)-2-(4-chlorobenzoylamino)-3-(2(1h)-quinolinon-4-yl)-propionic Acid
119. 2-[(4-chlorophenyl)formamido]-3-(2-oxo-1,2-dihydroquinolin-4-yl)propanoic Acid
120. 4-quinolinepropanoic Acid, .alpha.-((4-chlorobenzoyl)amino)-1,2-dihydro-2-oxo-
| Molecular Weight | 370.8 g/mol |
|---|---|
| Molecular Formula | C19H15ClN2O4 |
| XLogP3 | 2.4 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 5 |
| Exact Mass | 370.0720347 g/mol |
| Monoisotopic Mass | 370.0720347 g/mol |
| Topological Polar Surface Area | 95.5 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 598 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Antioxidants
Naturally occurring or synthetic substances that inhibit or retard oxidation reactions. They counteract the damaging effects of oxidation in animal tissues. (See all compounds classified as Antioxidants.)
Anti-Ulcer Agents
Various agents with different action mechanisms used to treat or ameliorate PEPTIC ULCER or irritation of the gastrointestinal tract. This has included ANTIBIOTICS to treat HELICOBACTER INFECTIONS; HISTAMINE H2 ANTAGONISTS to reduce GASTRIC ACID secretion; and ANTACIDS for symptomatic relief. (See all compounds classified as Anti-Ulcer Agents.)
A - Alimentary tract and metabolism
A02 - Drugs for acid related disorders
A02B - Drugs for peptic ulcer and gastro-oesophageal reflux disease (gord)
A02BX - Other drugs for peptic ulcer and gastro-oesophageal reflux disease (gord)
A02BX14 - Rebamipide
