


1. Ektebin
2. Peteha
3. Prothionamide
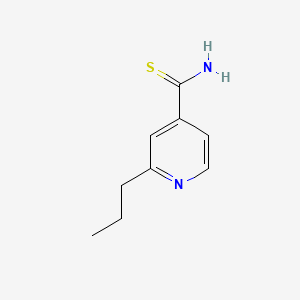
1. Prothionamide
2. 14222-60-7
3. 2-propylpyridine-4-carbothioamide
4. Ektebin
5. Protionamid
6. Trevintix
7. 2-propylthioisonicotinamide
8. Peteha
9. 2-propyl-thioisonicotinamide
10. Tuberex
11. Protionamidum
12. Protionizina
13. Tebeform
14. 2-propylisonicotinylthioamide
15. 4-pyridinecarbothioamide, 2-propyl-
16. Th-1321
17. 2-propyl-4-pyridinecarbothioamide
18. Rp 9778
19. 2-propyl-4-thiocarbamoylpyridine
20. Isonicotinamide, 2-propylthio-
21. 9778 R.p.
22. 1321 Th
23. Rp-9778
24. Protionamide (inn)
25. Prothionamidum
26. Nsc-758962
27. Protionamide (prothionamide)
28. Mls000042521
29. Protionamida
30. 76yoo33643
31. Ncgc00095164-01
32. Smr000047660
33. Protionamide 100 Microg/ml In Acetonitrile
34. Protionamide [inn]
35. Dsstox_cid_25940
36. Dsstox_rid_81238
37. Dsstox_gsid_45940
38. Protionamidum [inn-latin]
39. Protionamida [inn-spanish]
40. Trevintix (tn)
41. Cas-14222-60-7
42. Th 1321
43. Sr-05000001518
44. Einecs 238-093-7
45. Brn 0118164
46. Protion
47. Protionamide [inn:ban:dcf]
48. Unii-76yoo33643
49. Prothionamide (jp17)
50. Opera_id_999
51. Spectrum2_000019
52. Spectrum3_001964
53. Protionamide [mi]
54. Prothionamide [jan]
55. Schembl74572
56. Bspbio_003564
57. Protionamide [mart.]
58. 5-22-02-00376 (beilstein Handbook Reference)
59. Mls001201789
60. Mls006011877
61. Protionamide [who-dd]
62. Protionamide [who-ip]
63. Spectrum1505316
64. Spbio_000057
65. Chembl1378024
66. Dtxsid7045940
67. Chebi:32066
68. Kbio3_002911
69. Prothionamide, >=99% (hplc)
70. 2-propyl-4-thiocarbamoyl Pyridine
71. Hms1922d06
72. Hms2090j11
73. Hms2235m12
74. Hms3372k04
75. Hms3655o18
76. Hms3715g13
77. Kuc109576n
78. Pharmakon1600-01505316
79. Albb-010476
80. Bcp13522
81. Hy-b0306
82. Ksc-27-052d
83. Zinc3874803
84. Tox21_111463
85. Bbl010291
86. Bdbm50499814
87. Ccg-40049
88. Mfcd00464119
89. Nsc758962
90. Protionamidum [who-ip Latin]
91. S1881
92. Stk366469
93. Stl454225
94. Akos005172678
95. Tox21_111463_1
96. 1321-th
97. Ac-4518
98. Db12667
99. Ks-1282
100. Nsc 758962
101. 2-propylpyridine-4-carbimidothioic Acid
102. Ncgc00095164-02
103. Ncgc00095164-03
104. Ncgc00095164-04
105. Ncgc00095164-05
106. Sbi-0207058.p001
107. Db-042615
108. Ft-0630412
109. P2302
110. Sw199462-2
111. D01195
112. D88012
113. Ab00393463-12
114. Ab01093435-02
115. Ab01093435_03
116. Ab01093435_04
117. A807874
118. Q866657
119. Q-201638
120. Sr-05000001518-1
121. Sr-05000001518-2
122. Sr-05000001518-3
123. Brd-k75360161-001-09-9
| Molecular Weight | 180.27 g/mol |
|---|---|
| Molecular Formula | C9H12N2S |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 3 |
| Exact Mass | 180.07211956 g/mol |
| Monoisotopic Mass | 180.07211956 g/mol |
| Topological Polar Surface Area | 71 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 159 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antitubercular Agents
Drugs used in the treatment of tuberculosis. They are divided into two main classes: "first-line" agents, those with the greatest efficacy and acceptable degrees of toxicity used successfully in the great majority of cases; and "second-line" drugs used in drug-resistant cases or those in which some other patient-related condition has compromised the effectiveness of primary therapy. (See all compounds classified as Antitubercular Agents.)
J - Antiinfectives for systemic use
J04 - Antimycobacterials
J04A - Drugs for treatment of tuberculosis
J04AD - Thiocarbamide derivatives
J04AD01 - Protionamide
