



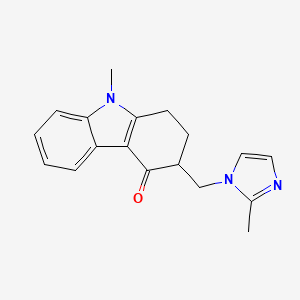

1. 4h-carbazol-4-one, 1,2,3,9-tetrahydro-9-methyl-3-((2-methyl-1h-imidazol-1-yl)methyl)-
2. Dihydrate, Ondansetron Monohydrochloride
3. Gr 38032f
4. Gr-38032f
5. Gr38032f
6. Hydrochloride, Ondansetron
7. Monohydrochloride Dihydrate, Ondansetron
8. Monohydrochloride, Ondansetron
9. Odt, Zofran
10. Ondansetron Hydrochloride
11. Ondansetron Monohydrochloride
12. Ondansetron Monohydrochloride Dihydrate
13. Ondansetron, (+,-)-isomer
14. Ondansetron, (r)-isomer
15. Ondansetron, (s)-isomer
16. Sn 307
17. Sn-307
18. Sn307
19. Zofran
20. Zofran Odt
1. 99614-02-5
2. Zofran
3. Zudan
4. 9-methyl-3-((2-methyl-1h-imidazol-1-yl)methyl)-2,3-dihydro-1h-carbazol-4(9h)-one
5. Zofran Odt
6. Zuplenz
7. 116002-70-1
8. 9-methyl-3-[(2-methylimidazol-1-yl)methyl]-2,3-dihydro-1h-carbazol-4-one
9. Gr 38032
10. A04aa01
11. 4af302esos
12. 9-methyl-3-[(2-methyl-1h-imidazol-1-yl)methyl]-2,3,4,9-tetrahydro-1h-carbazol-4-one
13. Chebi:7773
14. 99614-01-4
15. Eur-1025
16. 1,2,3,9-tetrahydro-9-methyl-3-((2-methyl-1h-imidazol-1-yl)methyl)-4h-carbazol-4-one
17. 9-methyl-3-[(2-methyl-1h-imidazol-1-yl)methyl]-1,2,3,9-tetrahydro-4h-carbazol-4-one
18. Nsc-757870
19. Ncgc00179341-02
20. Dsstox_cid_3393
21. 1,2,3,9-tetrahydro-9-methyl-3-[(2-methyl-1h-imidazol-1-yl)methyl]-4h-carbazol-4-one
22. 9-methyl-3-((2-methyl-1h-imidazol-1-yl)methyl)-1,2,3,9-tetrahydro-4h-carbazol-4-one
23. Dsstox_rid_77011
24. Dsstox_gsid_23393
25. Gr 38032 (hydrochloride);sn 307 (hydrochloride)
26. Ondansetron (zofran)
27. Gr 38032x
28. Cas-99614-02-5
29. Ondansetron Injection
30. Zofran Odt (tn)
31. Gr-c507/75
32. Desmethylondansetron
33. Unii-4af302esos
34. Brn 3622981
35. Ondansetron (jan/usp/inn)
36. Ondansetron [usp:inn:ban]
37. Sr-01000763250
38. Ondansetron (base And/or Unspecified Salts)
39. Chembl46
40. Ondansetron [mi]
41. 9-methyl-3-((2-methyl-1h-imidazol-1-yl)methyl)-1,2,3,9-tetrahydro-4h-carbazol-4-one Hydrochloride Dihydrate
42. Prestwick0_001058
43. Prestwick1_001058
44. Prestwick2_001058
45. Prestwick3_001058
46. Ondansetron [inn]
47. Ondansetron [jan]
48. Odansetron [common Misspelling Of Ondansetron]
49. Ondansetron [vandf]
50. Schembl4542
51. Timtec1_001750
52. (rs)-1,2,3,9-tetrahydro-9-methyl-3-(2-methylimidazol-1-ylmethyl)carbazol-4-one
53. Ondansetron [mart.]
54. Oprea1_435466
55. Oprea1_852372
56. Bspbio_001016
57. Cbdive_008994
58. Ondansetron [usp-rs]
59. Ondansetron [who-dd]
60. Mls006011928
61. Spbio_002938
62. Bpbio1_001118
63. Gtpl2290
64. Dtxsid8023393
65. Bdbm85330
66. Hsdb 8304
67. Hy-b0002b
68. Ondansetron [orange Book]
69. (3s)-9-methyl-3-[(2-methylimidazol-1-yl)methyl]-2,3-dihydro-1h-carbazol-4-one
70. Hms2090h16
71. Hms3259h08
72. Hms3371e18
73. Ondansetron [usp Monograph]
74. Act02589
75. Bcp28911
76. Tox21_113048
77. Cas_68647
78. Mfcd00833882
79. Nsc_68647
80. Nsc791005
81. S1996
82. Stk370548
83. Akos000599484
84. Akos016340526
85. Tox21_113048_1
86. Bcp9001025
87. Db00904
88. Gr38032
89. Ks-5227
90. Nc00706
91. Nsc 757870
92. Nsc-791005
93. 1,2,3,4-tetrahydro-9-methyl-3-(2-methyl-1h-imidazol-1-ylmethyl)carbazol-4-one
94. 1,2,3,9-tetrahydro-9-methyl-3-[(2-methyl-1h-imidazol-1-yl)methyl]-4h- Carbazol-4-one
95. 4h-carbazol-4-one, 1,2,3,9-tetrahydro-9-methyl-3-((2-methyl-1h-imidazol-1-yl)methyl)-
96. Ncgc00179341-04
97. Ncgc00179341-07
98. Ncgc00179341-12
99. Ac-28927
100. Gr-38032
101. Nci60_022780
102. Smr001307702
103. Gr-38032f/gr-38032
104. Ondansetron(base And/or Unspecified Salts)
105. Gr 38032f;gr-c507/75
106. Ab00373674
107. Ft-0631004
108. 39o049
109. C07325
110. D00456
111. Ab00373674-15
112. Ab00373674-17
113. Ab00373674_18
114. Ab00373674_19
115. L000456
116. Q410011
117. Sr-01000763250-4
118. Brd-a19736161-001-01-8
119. Brd-a19736161-003-03-0
120. Z1741971217
121. (+/-)-2,3-dihydro-9-methyl-3-((2-methylimidazol-1-yl)methyl)carbazol-4(1h)-one
122. 1,2,3,9,-tetrahydro-9-methyl-3-[(2-methyl-1h-imidazol-1-yl)methyl]-4h-carbazol-4-one
123. 1,2,3,9-tetrahydro-9-methyl-3-[(2-methyl-1h-imidazol-1yl)methyl]-4h-carbazol-4-one
124. 1,2,3,9-tetrahydro-9-methyl-3-[(2-methyl-1h-imidazole-1 -yl)methyl]-4h-carbazol-4-one
125. 1,2,3,9-tetrahydro-9-methyl-3-[(2-methyl-1h-imidazole-1-yl)methyl]-4h-carbazol-4-one
126. 4h-carbazol-4-one,?1,2,3,9-tetrahydro-9-methyl-3-[(2-methyl-1h-imidazol-1-yl)methyl]-
127. 9-methyl-3- (2-methyl-imidazol-1ylmethyl)-1,2,3,9-tetrahydro-carbazol-4-one
128. 9-methyl-3-[(2-methyl-1h-imidazolyl)methyl]-1,2,3,9-tetrahydro-4h-carbazole-4-one
129. 9-methyl-3-[(2-methylimidazol-1-yl)methyl]-2,3-dihydro-1~{h}-carbazol-4-one
130. 4h-carbazol-4-one, 1,2,3,9-tetrahydro-9-methyl-3-((2-methyl-1h-imidazol-1-yl)methyl)- (+/-)-
| Molecular Weight | 293.4 g/mol |
|---|---|
| Molecular Formula | C18H19N3O |
| XLogP3 | 2.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 293.152812238 g/mol |
| Monoisotopic Mass | 293.152812238 g/mol |
| Topological Polar Surface Area | 39.8 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 440 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 8 | |
|---|---|
| Drug Name | Ondansetron |
| PubMed Health | Ondansetron |
| Drug Classes | Antiemetic |
| Drug Label | DESCRIPTIONThe active ingredient in ondansetron hydrochloride tablets is ondansetron hydrochloride (HCl) USP as the dihydrate, the racemic form of ondansetron and a selective blocking agent of the serotonin 5-HT3 receptor type. Chemically it is ()... |
| Active Ingredient | Ondansetron |
| Dosage Form | Injectable; Tablet, orally disintegrating |
| Route | injection; Oral |
| Strength | 8mg; 4mg/2ml; 4mg |
| Market Status | Prescription |
| Company | Ranbaxy; Teva; Aurobindo Pharma; Sun Pharm Inds; Sandoz; Glenmark Generics; Luitpold; Mylan; Barr |
| 2 of 8 | |
|---|---|
| Drug Name | Zofran |
| PubMed Health | Ondansetron (Injection) |
| Drug Classes | Antiemetic |
| Drug Label | The active ingredient in ZOFRAN Tablets and ZOFRAN Oral Solution is ondansetron hydrochloride (HCl) as the dihydrate, the racemic form of ondansetron and a selective blocking agent of the serotonin 5-HT3 receptor type. Chemically it is (1, 2, 3, 9-te... |
| Active Ingredient | Ondansetron hydrochloride |
| Dosage Form | Tablet; Injectable; Solution |
| Route | Injection; Oral |
| Strength | eq 4mg base; eq 2mg base/ml; eq 4mg base/5ml; eq 24mg base; eq 8mg base |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 3 of 8 | |
|---|---|
| Drug Name | Zofran odt |
| PubMed Health | Ondansetron |
| Drug Classes | Antiemetic |
| Drug Label | The active ingredient in ZOFRAN Tablets and ZOFRAN Oral Solution is ondansetron hydrochloride (HCl) as the dihydrate, the racemic form of ondansetron and a selective blocking agent of the serotonin 5-HT3 receptor type. Chemically it is () 1, 2,... |
| Active Ingredient | Ondansetron |
| Dosage Form | Tablet, orally disintegrating |
| Route | Oral |
| Strength | 8mg; 4mg |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 4 of 8 | |
|---|---|
| Drug Name | Zuplenz |
| PubMed Health | Ondansetron (By mouth) |
| Drug Classes | Antiemetic |
| Drug Label | ZUPLENZ (ondansetron) oral soluble film is a white opaque orally dissolving film designed to be applied on top of the tongue where it will dissolve in 4 to 20 seconds and then is swallowed with saliva.ZUPLENZ does not require water to aid dissolution... |
| Active Ingredient | Ondansetron |
| Dosage Form | Film |
| Route | Oral |
| Strength | 8mg; 4mg |
| Market Status | Prescription |
| Company | Galena Biopharma |
| 5 of 8 | |
|---|---|
| Drug Name | Ondansetron |
| PubMed Health | Ondansetron |
| Drug Classes | Antiemetic |
| Drug Label | DESCRIPTIONThe active ingredient in ondansetron hydrochloride tablets is ondansetron hydrochloride (HCl) USP as the dihydrate, the racemic form of ondansetron and a selective blocking agent of the serotonin 5-HT3 receptor type. Chemically it is ()... |
| Active Ingredient | Ondansetron |
| Dosage Form | Injectable; Tablet, orally disintegrating |
| Route | injection; Oral |
| Strength | 8mg; 4mg/2ml; 4mg |
| Market Status | Prescription |
| Company | Ranbaxy; Teva; Aurobindo Pharma; Sun Pharm Inds; Sandoz; Glenmark Generics; Luitpold; Mylan; Barr |
| 6 of 8 | |
|---|---|
| Drug Name | Zofran |
| PubMed Health | Ondansetron (Injection) |
| Drug Classes | Antiemetic |
| Drug Label | The active ingredient in ZOFRAN Tablets and ZOFRAN Oral Solution is ondansetron hydrochloride (HCl) as the dihydrate, the racemic form of ondansetron and a selective blocking agent of the serotonin 5-HT3 receptor type. Chemically it is (1, 2, 3, 9-te... |
| Active Ingredient | Ondansetron hydrochloride |
| Dosage Form | Tablet; Injectable; Solution |
| Route | Injection; Oral |
| Strength | eq 4mg base; eq 2mg base/ml; eq 4mg base/5ml; eq 24mg base; eq 8mg base |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 7 of 8 | |
|---|---|
| Drug Name | Zofran odt |
| PubMed Health | Ondansetron |
| Drug Classes | Antiemetic |
| Drug Label | The active ingredient in ZOFRAN Tablets and ZOFRAN Oral Solution is ondansetron hydrochloride (HCl) as the dihydrate, the racemic form of ondansetron and a selective blocking agent of the serotonin 5-HT3 receptor type. Chemically it is () 1, 2,... |
| Active Ingredient | Ondansetron |
| Dosage Form | Tablet, orally disintegrating |
| Route | Oral |
| Strength | 8mg; 4mg |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 8 of 8 | |
|---|---|
| Drug Name | Zuplenz |
| PubMed Health | Ondansetron (By mouth) |
| Drug Classes | Antiemetic |
| Drug Label | ZUPLENZ (ondansetron) oral soluble film is a white opaque orally dissolving film designed to be applied on top of the tongue where it will dissolve in 4 to 20 seconds and then is swallowed with saliva.ZUPLENZ does not require water to aid dissolution... |
| Active Ingredient | Ondansetron |
| Dosage Form | Film |
| Route | Oral |
| Strength | 8mg; 4mg |
| Market Status | Prescription |
| Company | Galena Biopharma |
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Ondansetron is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of March 17, 2016: https://clinicaltrials.gov/ct2/results?term=Ondansetron&Search=Search
Prevention of nausea and vomiting associated with highly emetogenic cancer chemotherapy, including cisplatin ... . /Included in US product label/
NIH; DailyMed. Current Medication Information for Zofran (Ondansetron Hydrochloride) Solution; Zofran ODT (Ondansetron) Table, Orally Disintegrating; Zofran (Ondansetron Hydrochloride) Tablet, Film Coated (Updated: October 2014). Available from, as of January 20, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c7d61d98-fe86-4340-9b86-47eb92acaa0e
Prevention of nausea and vomiting associated with initial and repeat courses of moderately emetogenic cancer chemotherapy. /Included in US product label/
NIH; DailyMed. Current Medication Information for Zofran (Ondansetron Hydrochloride) Solution; Zofran ODT (Ondansetron) Table, Orally Disintegrating; Zofran (Ondansetron Hydrochloride) Tablet, Film Coated (Updated: October 2014). Available from, as of January 20, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c7d61d98-fe86-4340-9b86-47eb92acaa0e
Prevention of nausea and vomiting associated with radiotherapy in patients receiving either total body irradiation, single high-dose fraction to the abdomen, or daily fractions to the abdomen. /Included in US product label/
NIH; DailyMed. Current Medication Information for Zofran (Ondansetron Hydrochloride) Solution; Zofran ODT (Ondansetron) Table, Orally Disintegrating; Zofran (Ondansetron Hydrochloride) Tablet, Film Coated (Updated: October 2014). Available from, as of January 20, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c7d61d98-fe86-4340-9b86-47eb92acaa0e
For more Therapeutic Uses (Complete) data for Ondansetron (7 total), please visit the HSDB record page.
Because of the risk of QT-interval prolongation, ondansetron should be avoided in patients with congenital long QT syndrome. ECG monitoring is recommended in patients with electrolyte abnormalities such as hypokalemia or hypomagnesemia, congestive heart failure, or bradyarrhythmias and in those receiving other drugs known to prolong the QT interval. Electrolyte abnormalities should be corrected prior to IV administration of ondansetron. Because effects of ondansetron on the QT interval are dose related, use of single IV doses exceeding 16 mg should be avoided. Patients receiving ondansetron should be advised to seek immediate medical care if feelings of faintness, lightheadedness, irregular heartbeat, shortness of breath, or dizziness occur.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2910
Based on reports of profound hypotension and loss of consciousness when apomorphine was administered with ondansetron, concomitant use of apomorphine with ondansetron is contraindicated
NIH; DailyMed. Current Medication Information for Zofran (Ondansetron Hydrochloride) Solution; Zofran ODT (Ondansetron) Table, Orally Disintegrating; Zofran (Ondansetron Hydrochloride) Tablet, Film Coated (Updated: October 2014). Available from, as of January 21, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c7d61d98-fe86-4340-9b86-47eb92acaa0e
Advise patients of the possibility of serotonin syndrome with concomitant use of Zofran and another serotonergic agent such as medications to treat depression and migraines. Advise patients to seek immediate medical attention if the following symptoms occur: changes in mental status, autonomic instability, neuromuscular symptoms with or without gastrointestinal symptoms.
NIH; DailyMed. Current Medication Information for Zofran (Ondansetron Hydrochloride) Solution; Zofran ODT (Ondansetron) Table, Orally Disintegrating; Zofran (Ondansetron Hydrochloride) Tablet, Film Coated (Updated: October 2014). Available from, as of January 21, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c7d61d98-fe86-4340-9b86-47eb92acaa0e
Seizures (including tonic-clonic seizures) have been reported rarely in patients receiving ondansetron.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2909
For more Drug Warnings (Complete) data for Ondansetron (32 total), please visit the HSDB record page.
In the adult patient population: i) orally administered ondansetron tablets and orally disintegrating tablets (ODT) are indicated for: - the prevention of nausea and vomiting associated with emetogenic cancer chemotherapy, including high dose (ie. greater than or equal to 50 mg/m2) cisplatin therapy, and radiotherapy, and - the prevention and treatment of postoperative nausea and vomiting ii) intravenously administered ondansetron injection formulations are indicated for: - the prevention of nausea and vomiting associated with emetogenic cancer chemotherapy, including high dose (ie. greater than or equal to 50 mg/m2) cisplatin therapy, and - the prevention and treatment of postoperative nausea and vomiting In the pediatric (4-18 years of age) patient population: i) ondansetron was effective and well tolerated when given to children 4-12 years of age for the treatment of post-chemotherapy induced nausea and vomiting, ii) ondansetron tablets, ondansetron ODT, ondansetron injection are not indicated for the treatment of children 3 years of age or younger, iii) ondansetron tablets, ondansetron ODT, ondansetron injection are not indicated for use in any age group of the pediatric population for the treatment of post-radiotherapy induced nausea and vomiting, and iV) ondansetron tablets, ondansetron ODT, ondansetron injection are not indicated for use in any age group of the pediatric population for the treatment of postoperative nausea and vomiting In the geriatric (>65 years of age) patient population: i) efficacy and tolerance of ondansetron were similar to that observed in younger adults for the treatment of post-chemotherapy and radiotherapy-induced nausea and vomiting, and ii) clinical experience in the use of ondansetron in the prevention and treatment of postoperative nausea and vomiting is limited and is not indicated for use in the geriatric patient population
FDA Label
Ondansetron is a highly specific and selective serotonin 5-HT3 receptor antagonist, not shown to have activity at other known serotonin receptors and with low affinity for dopamine receptors,. The serotonin 5-HT3 receptors are located on the nerve terminals of the vagus in the periphery, and centrally in the chemoreceptor trigger zone of the area postrema,. The temporal relationship between the emetogenic action of emetogenic drugs and the release of serotonin, as well as the efficacy of antiemetic agents, suggest that chemotherapeutic agents release serotonin from the enterochromaffin cells of the small intestine by causing degenerative changes in the GI tract,. The serotonin then stimulates the vagal and splanchnic nerve receptors that project to the medullary vomiting center, as well as the 5-HT3 receptors in the area postrema, thus initiating the vomiting reflex, causing nausea and vomiting,. Moreover, the effect of ondansetron on the QTc interval was evaluated in a double-blind, randomized, placebo and positive (moxifloxacin) controlled, crossover study in 58 healthy adult men and women. Ondansetron was tested at single doses of 8 mg and 32 mg infused intravenously over 15 minutes. At the highest tested dose of 32 mg, prolongation of the Fridericia-corrected QTc interval (QT/RR0.33=QTcF) was observed from 15 min to 4 h after the start of the 15 min infusion, with a maximum mean (upper limit of 90% CI) difference in QTcF from placebo after baseline-correction of 19.6 (21.5) msec at 20 min. At the lower tested dose of 8 mg, QTc prolongation was observed from 15 min to 1 h after the start of the 15-minute infusion, with a maximum mean (upper limit of 90% CI) difference in QTcF from placebo after baseline-correction of 5.8 (7.8) msec at 15 min. The magnitude of QTc prolongation with ondansetron is expected to be greater if the infusion rate is faster than 15 minutes. The 32 mg intravenous dose of ondansetron must not be administered. No treatment-related effects on the QRS duration or the PR interval were observed at either the 8 or 32 mg dose. An ECG assessment study has not been performed for orally administered ondansetron. On the basis of pharmacokinetic-pharmacodynamic modelling, an 8 mg oral dose of ondansetron is predicted to cause a mean QTcF increase of 0.7 ms (90% CI -2.1, 3.3) at steady-state, assuming a mean maximal plasma concentration of 24.7 ng/mL (95% CI 21.1, 29.0). The magnitude of QTc prolongation at the recommended 5 mg/m2 dose in pediatrics has not been studied, but pharmacokinetic-pharmacodynamic modeling predicts a mean increase of 6.6 ms (90% CI 2.8, 10.7) at maximal plasma concentrations. In healthy subjects, single intravenous doses of 0.15 mg/kg of ondansetron had no effect on esophageal motility, gastric motility, lower esophageal sphincter pressure, or small intestinal transit time. Multiday administration of ondansetron has been shown to slow colonic transit in healthy subjects. Ondansetron has no effect on plasma prolactin concentrations.
Antiemetics
Drugs used to prevent NAUSEA or VOMITING. (See all compounds classified as Antiemetics.)
Antipruritics
Agents, usually topical, that relieve itching (pruritus). (See all compounds classified as Antipruritics.)
Serotonin 5-HT3 Receptor Antagonists
Drugs that bind to but do not activate SEROTONIN 5-HT3 RECEPTORS, thereby blocking the actions of SEROTONIN or SEROTONIN 5-HT3 RECEPTOR AGONISTS. (See all compounds classified as Serotonin 5-HT3 Receptor Antagonists.)
A04AA01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A04 - Antiemetics and antinauseants
A04A - Antiemetics and antinauseants
A04AA - Serotonin (5ht3) antagonists
A04AA01 - Ondansetron
Absorption
Ondansetron is absorbed from the gastrointestinal tract and undergoes some limited first-pass metabolism. Mean bioavailability in healthy subjects, following administration of a single 8-mg tablet, was recorded as being approximately 56% to 60%. Bioavailability is also slightly enhanced by the presence of food. Ondansetron systemic exposure does not increase proportionately to dose. The AUC from a 16-mg tablet was 24% greater than predicted from an 8-mg tablet dose. This may reflect some reduction of first-pass metabolism at higher oral doses.
Route of Elimination
Following oral or IV administration, ondansetron is extensively metabolised and excreted in the urine and faeces.
Volume of Distribution
The volume of distribution of ondansetron has been recorded as being approximately 160L.
Clearance
The clearance values determined for ondansetron in various patient age groups were recorded as approximately 0.38 L/h/kg in normal adult volunteers aged 19-40 yrs, 0.32 L/h/kg in normal adult volunteers aged 61-74 yrs, 0.26 L/h/kg in normal adult volunteers aged >=75 yrs.
Ondansetron is a 5-HT3 receptor antagonist that is an effective anti-emetic in cats. The purpose of this study was to evaluate the pharmacokinetics of ondansetron in healthy cats. Six cats with normal complete blood count, serum biochemistry, and urinalysis received 2 mg oral (mean 0.43 mg/kg), subcutaneous (mean 0.4 mg/kg), and intravenous (mean 0.4 mg/kg) ondansetron in a cross-over manner with a 5-day wash out. Serum was collected prior to, and at 0.25, 0.5, 1, 2, 4, 8, 12, 18, and 24 hr after administration of ondansetron. Ondansetron concentrations were measured using liquid chromatography coupled to tandem mass spectrometry. Noncompartmental pharmacokinetic modeling and dose interval modeling were performed. Repeated measures anova was used to compare parameters between administration routes. Bioavailability of ondansetron was 32% (oral) and 75% (subcutaneous). Calculated elimination half-life of ondansetron was 1.84 + or - 0.58 hr (intravenous), 1.18 + or - 0.27 hr (oral) and 3.17 + or - 0.53 hr (subcutaneous). The calculated elimination half-life of subcutaneous ondansetron was significantly longer (P < 0.05) than oral or intravenous administration. Subcutaneous administration of ondansetron to healthy cats is more bioavailable and results in a more prolonged exposure than oral administration. This information will aid management of emesis in feline patients.
PMID:24330064 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4059788 Quimby JM et al; J Vet Pharmacol Ther 37 (4): 348-53 (2014)
Nausea and vomiting are some of the major side effects caused by certain drug therapies, e.g. chemotherapy, radiotherapy and general anesthesia. Because of the nature of the symptoms, oral delivery is inappropriate, while intravenous administration may be unpractical. The aim of the present study was to develop a transdermal gel (2% Klucel) for ondansetron, a first line 5-HT3-receptor-antagonist antiemetic. The effects of the penetration enhancer camphor and isopropyl-myristate (IPM) were first investigated in-vitro using modified Franz diffusion-cells and then tested in-vivo in a rabbit model by measuring skin and plasma concentrations. Since a disadvantage of transdermal delivery is a prolonged lag-time, the effect of skin treatment with a micro-needle roller was tested. The in-vitro permeation studies through excised porcine ear skin showed that the presence of 2.5% camphor or IPM increased steady state flux by 1.2- and 2.5-fold, respectively, compared to the control gel. Ondansetron was not detectable in either skin or plasma following in-vivo application of the base-gel, whereas the camphor gel and IPM gel delivered 20 and 81 ug/sq cm of ondansetron, respectively. Microporation led to an increase in plasma Cmax and AUC by 10.47 + or - 1.68-fold and 9.31 + or - 4.91-fold, respectively, for the camphor gel, and by 2.31 + or -0.53-fold and 1.59 + or - 0.38-fold, respectively for the IPM gel. In conclusion, the 2.5% IPM gel demonstrated optimal in-vivo transdermal flux. Skin pretreatment with a micro-needle roller slightly improved the delivery of the IPM gel, whereas dramatically increased the transdermal delivery of the camphor gel.
PMID:24919508 Patel DR et al; Drug Dev Ind Pharm 41 (6): 1030-6 (2015)
Ondansetron is a potent antiemetic drug that has been commonly used to treat acute and chemotherapy-induced nausea and vomiting (CINV) in dogs. The aim of this study was to perform a pharmacokinetic analysis of ondansetron in dogs following oral administration of a single dose. A single 8-mg oral dose of ondansetron was administered to beagles (n = 18), and the plasma concentrations of ondansetron were measured by liquid chromatography-tandem mass spectrometry. The data were analyzed by modeling approaches using ADAPT5, and model discrimination was determined by the likelihood-ratio test. The peak plasma concentration (Cmax ) was 11.5 +/- 10.0 ng/mL at 1.1 +/- 0.8 hr. The area under the plasma concentration vs. time curve from time zero to the last measurable concentration was 15.9 +/- 14.7 ng hr/mL, and the half-life calculated from the terminal phase was 1.3 +/- 0.7 hr. The interindividual variability of the pharmacokinetic parameters was high (coefficient of variation > 44.1%), and the one-compartment model described the pharmacokinetics of ondansetron well. The estimated plasma concentration range of the usual empirical dose from the Monte Carlo simulation was 0.1-13.2 ng/mL. These findings will facilitate determination of the optimal dose regimen for dogs with CINV.
PMID:25131428 Baek IH et al; J Vet Pharmacol Ther 38 (2): 199-202 (2015)
Ondansetron is the drug of choice to prevent nausea in women undergoing cesarean surgery and can be used to prevent neonatal abstinence syndrome (NAS). The pharmacokinetics of ondansetron have not been characterized in pregnant women or in newborns. A nonlinear mixed-effects modeling approach was used to analyze plasma samples obtained from 20 nonpregnant and 40 pregnant women following a single administration of 4 or 8 mg ondansetron, from umbilical cord blood at delivery, and from neonates after birth. The analysis indicates that: ondansetron disposition is not affected by pregnancy (P > 0.05), but influenced by dose (P < 0.05), and is characterized by rapid transplacental transfer and longer elimination half-life in neonates compared to their mother. A dosing regimen for prevention of NAS was designed based on the model. The regimen involves IV administration of 4 mg to the mothers shortly before cord clamping, or oral administration of 0.07 mg/kg (or equivalently 0.04 mg/kg IV) to neonates.
PMID:25670522 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4325425 Elkomy MH et al; Clin Pharmacol Ther 97 (2): 167-76 (2015)
For more Absorption, Distribution and Excretion (Complete) data for Ondansetron (8 total), please visit the HSDB record page.
In vitro metabolism studies have shown that ondansetron is a substrate for human hepatic cytochrome P450 enzymes, including CYP1A2, CYP2D6 and CYP3A4. In terms of overall ondansetron turnover, CYP3A4 played the predominant role. Because of the multiplicity of metabolic enzymes capable of metabolizing ondansetron, it is likely that inhibition or loss of one enzyme (e.g. CYP2D6 enzyme deficiency) will be compensated by others and may result in little change in overall rates of ondansetron clearance. Following oral or IV administration, ondansetron is extensively metabolised and excreted in the urine and faeces. In humans, less than 10% of the dose is excreted unchanged in the urine. The major urinary metabolites are glucuronide conjugates (45%), sulphate conjugates (20%) and hydroxylation products (10%). The primary metabolic pathway is subsequently hydroxylation on the indole ring followed by subsequent glucuronide or sulfate conjugation. Although some nonconjugated metabolites have pharmacologic activity, these are not found in plasma at concentrations likely to significantly contribute to the biological activity of ondansetron.
Ondansetron is extensively metabolized in humans, with approximately 5% of a radiolabeled dose recovered as the parent compound from the urine. The primary metabolic pathway is hydroxylation on the indole ring followed by subsequent glucuronide or sulfate conjugation. Although some nonconjugated metabolites have pharmacologic activity, these are not found in plasma at concentrations likely to significantly contribute to the biological activity of ondansetron.
NIH; DailyMed. Current Medication Information for Zofran (Ondansetron Hydrochloride) Solution; Zofran ODT (Ondansetron) Table, Orally Disintegrating; Zofran (Ondansetron Hydrochloride) Tablet, Film Coated (Updated: October 2014). Available from, as of January 26, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c7d61d98-fe86-4340-9b86-47eb92acaa0e
Ondansetron has known human metabolites that include 6-hydroxy-ondansetron, 7-hydroxy-ondansetron, and 8-hydroxy-ondansetron.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The half-life of ondansetron after either an 8 mg oral dose or intravenous dose was approximately 3-4 hours and could be extended to 6-8 hours in the elderly.
In humans ... elimination half lives are approximately 3-4 hours, but are prolonged in elderly patients.
Plumb D.C. Veterinary Drug Handbook. 8th ed. (pocket). Ames, IA: Wiley-Blackwell, 2015., p. 1071
... Six cats with normal complete blood count, serum biochemistry, and urinalysis received 2 mg oral (mean 0.43 mg/kg), subcutaneous (mean 0.4 mg/kg), and intravenous (mean 0.4 mg/kg) ondansetron in a cross-over manner with a 5-day wash out. Serum was collected prior to, and at 0.25, 0.5, 1, 2, 4, 8, 12, 18, and 24 hr after administration of ondansetron. ... Calculated elimination half-life of ondansetron was 1.84 + or - 0.58 hr (intravenous), 1.18 + or - 0.27 hr (oral) and 3.17 + or - 0.53 hr (subcutaneous). The calculated elimination half-life of subcutaneous ondansetron was significantly longer (P < 0.05) than oral or intravenous administration. ...
PMID:24330064 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4059788 Quimby JM et al; J Vet Pharmacol Ther 37 (4): 348-53 (2014)
... A single 8-mg oral dose of ondansetron was administered to beagles (n = 18), ... and the half-life calculated from the terminal phase was 1.3 +/- 0.7 hr. ...
PMID:25131428 Baek IH et al; J Vet Pharmacol Ther 38 (2): 199-202 (2015)
Ondansetron is a selective antagonist of the serotonin receptor subtype, 5-HT3. Cytotoxic chemotherapy and radiotherapy are associated with the release of serotonin (5-HT) from enterochromaffin cells of the small intestine, presumably initiating a vomiting reflex through stimulation of 5-HT3 receptors located on vagal afferents. Ondansetron may block the initiation of this reflex. Activation of vagal afferents may also cause a central release of serotonin from the chemoreceptor trigger zone of the area postrema, located on the floor of the fourth ventricle. Thus, the antiemetic effect of ondansetron is probably due to the selective antagonism of 5-HT3 receptors on neurons located in either the peripheral or central nervous systems, or both. Although the mechanisms of action of ondansetron in treating postoperative nausea and vomiting and cytotoxic induced nausea and vomiting may share similar pathways, the role of ondansetron in opiate-induced emesis has not yet been formally established.