






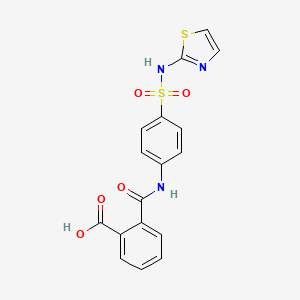

1. Ftalazol
2. Phthalazol
3. Phthalylsulfathiazole Monosodium Salt
1. 85-73-4
2. Sulfathalidine
3. Phthalylsulphathiazole
4. Phthalazol
5. Phthalylsulfonazole
6. Cremothalidine
7. Sulphaphthalyl
8. Phthalazole
9. Sulfaphthalazole
10. Phthalylnorsulfazole
11. Enteramida
12. Entexidina
13. Phtalazol
14. N4-phthalylsulfathiazole
15. Intestiazol
16. Phthalidin
17. Sulfacetil
18. Thalistanin
19. Thalistatyl
20. Ultratiazol
21. Ftalazol
22. Ftalysept
23. Sulftalyl
24. Taleudron
25. Talidine
26. Taloudron
27. Thalazole
28. Thalinil
29. Entero-sulfina
30. Ftalil-esteve
31. Ftalil-septol
32. Phthaloylsulfathiazole
33. Afi-ftalyl
34. Phtalylsulfathiazol
35. 4'-(2-thiazolylsulfamyl)phthalanilic Acid
36. Phthalylsulfathiazolum
37. 4'-(2-thiazolylsulfamoyl)phthalanilic Acid
38. Ftalilsulfatiazol
39. Ftalylsulfathiazol
40. 2-(p-n-phthalylsulfanilyl)aminothiazole
41. 2-(p-phthalylaminobenzenesulfamido)thiazole
42. 2-(n4-phthalylaminobenzenesulfonamide)thiazole
43. (o-carboxybenzoyl)-p-aminophenylsulfonamidothiazole
44. 2-((4-(n-(thiazol-2-yl)sulfamoyl)phenyl)carbamoyl)benzoic Acid
45. Phthalylsulfathiazole [inn]
46. Nsc 66454
47. Nsc 683525
48. 2-(n(sup4)-phthalylsulfanilamido)thiazole
49. 2-(n(sup 4)-phthalylsulfanilamido)thiazole
50. Nsc-66454
51. Phthalanilic Acid, 4'-(2-thiazolylsulfamoyl)-
52. 2-[[4-(1,3-thiazol-2-ylsulfamoyl)phenyl]carbamoyl]benzoic Acid
53. Nsc-683525
54. 2-(n(sup 4)-phthalyaminobenzenesulfonamide)thiazole
55. 2-(n(sup 4)-phthalyaminobenzenesulfonamido)thiazole
56. Mls002693575
57. Chebi:9336
58. 85-73-4 (free)
59. Phthalazolum
60. Sulfanilamide, N(sup 4)-(o-carboxybenzoyl)-n'-2-thiazolyl-
61. 2-{[4-(1,3-thiazol-2-ylsulfamoyl)phenyl]carbamoyl}benzoic Acid
62. 2-({4-[(1,3-thiazol-2-yl)sulfamoyl]phenyl}carbamoyl)benzoic Acid
63. Benzoic Acid, 2-(((4-((2-thiazolylamino)sulfonyl)phenyl)amino)carbonyl)-
64. Benzoic Acid, 2-[[[4-[(2-thiazolylamino)sulfonyl]phenyl]amino]carbonyl]-
65. Nsc66454
66. Nsc683525
67. Cas-85-73-4
68. Ncgc00016337-02
69. 2-[[4-(thiazol-2-ylsulfamoyl)phenyl]carbamoyl]benzoic Acid
70. Entexidin
71. 6875l5852v
72. Dsstox_cid_3470
73. Phthalylsulfathiazole (inn)
74. Dsstox_rid_77039
75. Dsstox_gsid_23470
76. Ftalilsofatiazolo
77. Ftalilsofatiazolo [dcit]
78. Phthalylsulfathiazole(pst)
79. 2-[({4-[(1,3-thiazol-2-ylamino)sulfonyl]phenyl}amino)carbonyl]benzoic Acid
80. Ftalylsulfathiazol [czech]
81. Smr001233366
82. Ftalilsulfatiazol [inn-spanish]
83. Phtalylsulfathiazol [inn-french]
84. Phthalylsulfathiazolum [inn-latin]
85. Sr-05000001793
86. Einecs 201-627-4
87. Ai3-18632
88. Sulfaphtalylthiazol
89. Phthalylsulfathiazol
90. Unii-6875l5852v
91. Prestwick_980
92. 2-(n4-phthalylsulfanilamido)thiazole
93. Phthalylsulfathiazole [usp:inn:ban]
94. Phthalylsulfhathiazole
95. Phthalylsulphathiozole
96. Spectrum_000047
97. Prestwick0_000869
98. Prestwick1_000869
99. Prestwick2_000869
100. Prestwick3_000869
101. Spectrum3_001486
102. Spectrum4_000154
103. Spectrum5_001147
104. 2-[[[4-[(2-thiazolylamino)sulfonyl]phenyl]amino]carbonyl]benzoic Acid
105. Component Of Sulfathalidine
106. Ec 201-627-4
107. Oprea1_620492
108. Bspbio_000917
109. Bspbio_003071
110. Kbiogr_000628
111. Kbioss_000427
112. Mls002154042
113. Divk1c_001029
114. Schembl152662
115. Spectrum1502021
116. Spbio_002838
117. Bpbio1_001009
118. Chembl1524273
119. Dtxsid8023470
120. Hms503m19
121. Kbio1_001029
122. Kbio2_000427
123. Kbio2_002995
124. Kbio2_005563
125. Kbio3_002571
126. Phthalylsulfathiazole [mi]
127. Ninds_001029
128. Hms1570n19
129. Hms1921d22
130. Hms2092n19
131. Hms2097n19
132. Hms2230g21
133. Hms3374a01
134. Hms3714n19
135. Pharmakon1600-01502021
136. Hy-b1407
137. Str05252
138. Zinc1530877
139. Tox21_110380
140. Mfcd00005318
141. Nsc758159
142. Phthalylsulfathiazole [mart.]
143. S5700
144. Stk730435
145. Wln: T5n Csj Bmswr Dmvr Bvq
146. Phthalylsulfathiazole [who-dd]
147. Akos000745948
148. Tox21_110380_1
149. Ccg-212977
150. Cs-4862
151. Db13248
152. Nsc-758159
153. Idi1_001029
154. Ncgc00016337-01
155. Ncgc00016337-03
156. Ncgc00016337-05
157. Ncgc00094943-01
158. Ncgc00094943-02
159. Ac-19044
160. Sbi-0051702.p002
161. Sbi-0051702.p003
162. Db-056881
163. Phthalylsulfathiazole [ep Monograph]
164. Ab00052257
165. Ft-0631402
166. T0703
167. 2-(n4-phthalylaminobenzenesulfonamido)thiazole
168. C07659
169. D02440
170. D78257
171. Ab00052257_08
172. A841437
173. Q4493189
174. Sr-05000001793-1
175. Sr-05000001793-3
176. Brd-k64659768-001-04-5
177. Sulfanilamide, N4-(o-carboxybenzoyl)-n'-2-thiazolyl-
178. 2-(4-(n-thiazol-2-ylsulfamoyl)phenylcarbamoyl)benzoic Acid
179. N4-phthalylsulfathiazole, Vetranal(tm), Analytical Standard
180. 2-((4-[(1,3-thiazol-2-ylamino)sulfonyl]anilino)carbonyl)benzoic Acid #
181. 2-[[[4-[(2-thiazolylamino)sulfonyl]phenyl]amino]carbonyl]-benzoic Acid
182. Phthalylsulfathiazole, European Pharmacopoeia (ep) Reference Standard
| Molecular Weight | 403.4 g/mol |
|---|---|
| Molecular Formula | C17H13N3O5S2 |
| XLogP3 | 1.1 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 6 |
| Exact Mass | 403.02966287 g/mol |
| Monoisotopic Mass | 403.02966287 g/mol |
| Topological Polar Surface Area | 162 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 643 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
A - Alimentary tract and metabolism
A07 - Antidiarrheals, intestinal antiinflammatory/antiinfective agents
A07A - Intestinal antiinfectives
A07AB - Sulfonamides
A07AB02 - Phthalylsulfathiazole
