





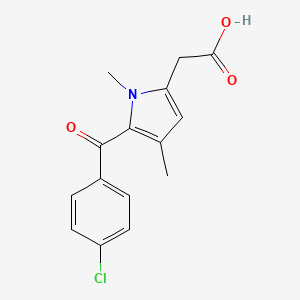

1. 5-(4-chlorobenzoyl)-1,4-dimethyl-1h-pyrrole-2-acetate Dihydrate
2. Mcn 2783-21-98
3. Zomax
4. Zomepirac Potassium
5. Zomepirac Sodium
1. 33369-31-2
2. 5-(4-chlorobenzoyl)-1,4-dimethyl-1h-pyrrole-2-acetic Acid
3. [5-(4-chlorobenzoyl)-1,4-dimethyl-1h-pyrrol-2-yl]acetic Acid
4. 2-[5-(4-chlorobenzoyl)-1,4-dimethylpyrrol-2-yl]acetic Acid
5. Chembl19490
6. Chebi:35859
7. 822g987u9j
8. 1h-pyrrole-2-aceticacid, 5-(4-chlorobenzoyl)-1,4-dimethyl-
9. Zomepiracum [inn-latin]
10. 1,4-dimethyl-5-p-chlorobenzoylpyrrole-2-acetic Acid
11. Zomepiracum
12. Zomepirac [inn:ban]
13. Ncgc00094811-01
14. Einecs 251-474-2
15. Sr-05000001739
16. {5-[(4-chlorophenyl)carbonyl]-1,4-dimethyl-1h-pyrrol-2-yl}acetic Acid
17. Unii-822g987u9j
18. Zom
19. Spectrum_000306
20. Zomepirac [inn]
21. Zomepirac [mi]
22. Prestwick0_000779
23. Prestwick1_000779
24. Prestwick2_000779
25. Prestwick3_000779
26. Spectrum2_000955
27. Spectrum3_000589
28. Spectrum4_000383
29. Spectrum5_001427
30. Zomepirac [who-dd]
31. Schembl25735
32. Bspbio_000858
33. Bspbio_002038
34. Kbiogr_000905
35. Kbioss_000786
36. Divk1c_000953
37. Spectrum1500615
38. Spbio_000950
39. Spbio_002797
40. Bpbio1_000944
41. Dtxsid9023754
42. Hms502p15
43. Kbio1_000953
44. Kbio2_000786
45. Kbio2_003354
46. Kbio2_005922
47. Kbio3_001538
48. Zinc57537
49. Zomepirac; Aif; Ce0; Corrdec
50. Ninds_000953
51. Hms1921k11
52. Hms2092e04
53. Pharmakon1600-01500615
54. 1h-pyrrole-2-acetic Acid, 5-(4-chlorobenzoyl)-1,4-dimethyl-
55. Bdbm50027952
56. Ccg-39056
57. Nsc757379
58. Db04828
59. Idi1_000953
60. Ncgc00094811-02
61. Ncgc00094811-03
62. Ncgc00094811-04
63. Da-06775
64. Sbi-0051557.p002
65. Ft-0708860
66. Ab00052126_03
67. Q4024685
68. Sr-05000001739-1
69. Brd-k81326768-001-02-4
70. Brd-k81326768-236-03-4
71. 5-(p-chlorobenzoyl)-1,4-dimethyl-pyrrole-2-acetic Acid
72. 5-(p-chlorobenzoyl)-1,4-dimethylpyrrole-2-acetic Acid
73. 5-(rho-chlorobenzoyl)-1,4-dimethylpyrrole-2-acetic Acid
| Molecular Weight | 291.73 g/mol |
|---|---|
| Molecular Formula | C15H14ClNO3 |
| XLogP3 | 2.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 291.0662210 g/mol |
| Monoisotopic Mass | 291.0662210 g/mol |
| Topological Polar Surface Area | 59.3 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 379 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Zomepirac was indicated for the management of mild to severe pain.
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
M - Musculo-skeletal system
M01 - Antiinflammatory and antirheumatic products
M01A - Antiinflammatory and antirheumatic products, non-steroids
M01AB - Acetic acid derivatives and related substances
M01AB04 - Zomepirac
Zomepirac has known human metabolites that include Zomepirac O-glucuronide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
