






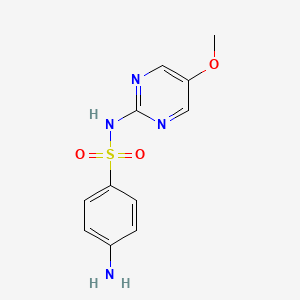

1. Sulfamethoxydiazine
2. Sulfamethoxydine
3. Sulfametin
4. Sulfametorinum
5. Sulfametoxidine
6. Sulfametoxydiazine
7. Sulphamethoxydiazine
1. 651-06-9
2. 5-methoxysulfadiazine
3. Sulfamethoxydiazine
4. Sulfametoxydiazine
5. Sulfamethoxine
6. Sulfametin
7. Sulfamethoxypyrimidine
8. 4-amino-n-(5-methoxypyrimidin-2-yl)benzenesulfonamide
9. Sulphamethoxydiazine
10. Ultrax
11. Sulla
12. Sulfametorine
13. Sulphameter
14. Methoxypyrimal
15. Sulfamethoxydin
16. Berlicid
17. Sulfa-5-methoxypyrimidine
18. Sulfamethorine
19. Longasulf
20. Supramid
21. Bayrena
22. Dairena
23. Durenat
24. Juvoxin
25. Kinecid
26. Kirocid
27. Kiron
28. Ahr-857
29. Solfametossidiazina
30. Sulfametoxipirimidine
31. Sulfameter [usan]
32. 2-sulfanilamido-5-methoxypyrimidine
33. 4-amino-n-(5-methoxy-2-pyrimidinyl)benzenesulfonamide
34. 5-methoxy-2-sulfanilamidopyrimidine
35. Sulfamethoxydiazin
36. Sulfametoxidiazina
37. Bayer 5400
38. Sulfametoxydiazinum
39. Benzenesulfonamide, 4-amino-n-(5-methoxy-2-pyrimidinyl)-
40. 2-(4-aminobenzenesulfonamido)-5-methoxypyrimidine
41. 2-sulfanilamido-5-methoxypyrimidin
42. Sulfamethoxidiazine
43. Sh 613
44. Nsc 683528
45. N(sup 1)-(5-methoxy-2-pyrimidinyl)sulfanilamide
46. Sulfameter (bayrena)
47. 4-amino-n-(5-methoxypyrimidin-2-yl)benzene-1-sulfonamide
48. Sulfametoxydiazine [inn]
49. I-2586
50. Nsc-683528
51. Nsc-757874
52. Chebi:53727
53. Bay-5400
54. Sulfameter (usan)
55. Nsc683528
56. Sh-613
57. Sulfanilamide, N(sup 1)-(5-methoxy-2-pyrimidinyl)-
58. 3l179f09d6
59. Sulfametoxydiazine;5-methoxysulfadiazine
60. Ncgc00016530-01
61. Sulfametinum
62. Benzenesulfonamide, 4-amino-n-(5-methoxy-2-pyridimidinyl)-
63. Cas-651-06-9
64. Sulfametoxydiazine (inn)
65. Dsstox_cid_3613
66. N(1)-(5-methoxy-2-pyrimidinyl)sulfanilamide
67. Dsstox_rid_77110
68. Dsstox_gsid_23613
69. Solfametossidiazina [dcit]
70. Sulfametoxydiazinum [inn-latin]
71. Sulfametoxidiazina [inn-spanish]
72. Sr-01000721917
73. Einecs 211-480-8
74. Brn 0621130
75. N1-(5-methoxy-2-pyrimidinyl)sulfanilamide
76. 2-sulfanilamido-5-methoxypyrimidin [german]
77. Unii-3l179f09d6
78. Mfcd00006067
79. Prestwick_1048
80. Sulla (tn)
81. Spectrum_001149
82. Sulfameter [mi]
83. Prestwick0_000769
84. Prestwick1_000769
85. Prestwick2_000769
86. Prestwick3_000769
87. Spectrum2_001428
88. Spectrum3_001463
89. Spectrum4_000426
90. Spectrum5_000981
91. 2-sulfa-5-methoxypyrimidine
92. Oprea1_482593
93. Schembl79417
94. Bspbio_000818
95. Bspbio_002985
96. Kbiogr_000752
97. Kbioss_001629
98. 5-25-12-00525 (beilstein Handbook Reference)
99. Mls000069640
100. Bidd:gt0693
101. Divk1c_000468
102. Spectrum1501155
103. Spbio_001536
104. Spbio_002757
105. Sulfamethoxydiazine / Sulfamete
106. Bpbio1_000900
107. Chembl1200359
108. Dtxsid5023613
109. Sulfameter [orange Book]
110. Gptonymqftzpkc-uhfffaoysa-
111. Hms501h10
112. Kbio1_000468
113. Kbio2_001629
114. Kbio2_004197
115. Kbio2_006765
116. Kbio3_002485
117. Zinc49142
118. Ninds_000468
119. Hms1570i20
120. Hms1921l15
121. Hms2092j03
122. Hms2097i20
123. Hms2233b12
124. Hms3374j11
125. Hms3655a11
126. Hms3714i20
127. Pharmakon1600-01501155
128. Sulfametoxydiazine [who-dd]
129. Amy38159
130. Hy-b0213
131. Tox21_110478
132. Ccg-38965
133. Nsc757874
134. S1618
135. Akos015897255
136. Sulfameter 100 Microg/ml In Methanol
137. Tox21_110478_1
138. Db06821
139. Idi1_000468
140. Ncgc00016530-02
141. Ncgc00016530-03
142. Ncgc00016530-04
143. Ncgc00016530-06
144. Ncgc00016530-07
145. Ncgc00094915-01
146. Ncgc00094915-02
147. Ac-19932
148. Ac-32614
149. As-13912
150. Smr000058215
151. Sbi-0051665.p002
152. Db-054761
153. Ab00052227
154. Ft-0632748
155. Sw197118-3
156. 51s069
157. D02517
158. Sulfameter, Vetranal(tm), Analytical Standard
159. Ab00052227-11
160. Ab00052227_12
161. Ab00052227_13
162. A834976
163. Sr-01000721917-2
164. Sr-01000721917-3
165. W-104807
166. Brd-k87492696-001-05-8
167. Brd-k87492696-001-09-0
168. Q15410181
169. 4-azanyl-n-(5-methoxypyrimidin-2-yl)benzenesulfonamide
170. Z1522567176
171. 4-amino-n-[5-(methyloxy)pyrimidin-2-yl]benzenesulfonamide
| Molecular Weight | 280.31 g/mol |
|---|---|
| Molecular Formula | C11H12N4O3S |
| XLogP3 | 0.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 4 |
| Exact Mass | 280.06301143 g/mol |
| Monoisotopic Mass | 280.06301143 g/mol |
| Topological Polar Surface Area | 116 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 368 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Infective Agents
Substances that prevent infectious agents or organisms from spreading or kill infectious agents in order to prevent the spread of infection. (See all compounds classified as Anti-Infective Agents.)
Leprostatic Agents
Substances that suppress Mycobacterium leprae, ameliorate the clinical manifestations of leprosy, and/or reduce the incidence and severity of leprous reactions. (See all compounds classified as Leprostatic Agents.)
Anti-Infective Agents, Urinary
Substances capable of killing agents causing urinary tract infections or of preventing them from spreading. (See all compounds classified as Anti-Infective Agents, Urinary.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01E - Sulfonamides and trimethoprim
J01ED - Long-acting sulfonamides
J01ED04 - Sulfametoxydiazine
