




1. Cortisdin
2. Efflumidex
3. Flucon
4. Flucon, Isopto
5. Fluor Op
6. Fluor-op
7. Fluoro Ophtal
8. Fluoro-ophtal
9. Fluoropos
10. Fml
11. Fml Forte
12. Fml Liquifilm
13. Isopto Flucon
14. Pms Fluorometholone
15. Pms-fluorometholone
1. 426-13-1
2. Fluoromethalone
3. Oxylone
4. Flumetholon
5. Fluor-op
6. Fluormetholone
7. Cortilet
8. Delmeson
9. Fml Liquifilm
10. Trilcin
11. Fml Forte
12. Fluorometolona
13. Fluorometholonum
14. Fluormetholon
15. Nsc 33001
16. Fml
17. Component Of Neo-oxylone
18. U 8614
19. Chebi:31625
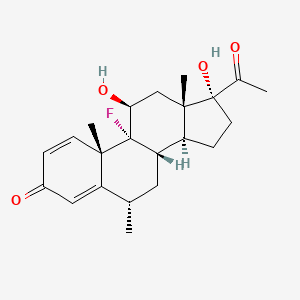
20. 9-fluoro-11beta,17-dihydroxy-6alpha-methylpregna-1,4-diene-3,20-dione
21. Nsc-33001
22. (6s,8s,9r,10s,11s,13s,14s,17r)-17-acetyl-9-fluoro-11,17-dihydroxy-6,10,13-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one
23. Sv0csg527l
24. Mls000069537
25. Mls001076157
26. Fluormetholonum
27. 9-fluoro-11,17-dihydroxy-6-methylpregna-1,4-diene-3,20-dione
28. Nsc33001
29. Fluorometolone
30. Smr000058598
31. Fluorometolone [dcit]
32. Neo-oxylone
33. Fml-s Liquifilm
34. Dsstox_cid_27435
35. Dsstox_rid_82345
36. Fml S.o.p.
37. Dsstox_gsid_47435
38. Fluorometholonum [inn-latin]
39. Fluorometolona [inn-spanish]
40. Pregna-1,4-diene-3,20-dione, 9-fluoro-11,17-dihydroxy-6-methyl-, (6.alpha.,11.beta.)-
41. Oxylone (tn)
42. Fluor-op (tn)
43. Fml (tn)
44. Einecs 207-041-5
45. Unii-sv0csg527l
46. Fluorometholon
47. Ai3-52813
48. Ncgc00016442-01
49. Cas-426-13-1
50. Prestwick_227
51. 9-fluoro-11-beta,17-dihydroxy-6-alpha-methylpregna-1,4-diene-3,20-dione
52. Fluorometholone [usp:inn:ban:jan]
53. Mfcd00056461
54. Opera_id_341
55. Prestwick0_000718
56. Prestwick1_000718
57. Prestwick2_000718
58. Prestwick3_000718
59. F0414
60. 11beta,17alpha-dihydroxy-9-fluoro-6-methyl-1,4-pregnadiene-3,20-dione
61. Fluorometholone, >=98%
62. Pregna-1,4-diene-3,20-dione, 9-fluoro-11-beta,17-dihydroxy-6-alpha-methyl-
63. Schembl5051
64. Fluorometholone [mi]
65. Pregna-1,4-diene-3,20-dione, 9-fluoro-11,17-dihydroxy-6-methyl-, (6alpha,11beta)-
66. Bspbio_000935
67. Fluorometholone [inn]
68. Fluorometholone [jan]
69. Spbio_002856
70. Fluorometholone [vandf]
71. Bpbio1_001029
72. Gtpl7079
73. Fluorometholone [mart.]
74. Chembl1200600
75. Dtxsid7047435
76. Fluorometholone [usp-rs]
77. Fluorometholone [who-dd]
78. 9-fluoro-11.beta.,17-dihydroxy-6.alpha.-methylpregna-1,4-diene-3,20-dione
79. Pregna-1,4-diene-3,20-dione, 9-fluoro-11.beta.,17-dihydroxy-6.alpha.-methyl-
80. Fluorometholone (jp17/usp/inn)
81. Hms1570o17
82. Hms2097o17
83. Hms2234f16
84. Hms3714o17
85. Hy-b1893
86. Tox21_110440
87. Tox21_302593
88. Bdbm50103631
89. Fluorometholone [orange Book]
90. S5486
91. Akos015895108
92. Fml-s Component Fluorometholone
93. Pregna-1,4-diene-3,20-dione, 9-fluoro-11beta,17-dihydroxy-6alpha-methyl-
94. Tox21_110440_1
95. Zinc118912517
96. Ac-3520
97. Ccg-220718
98. Db00324
99. Fluorometholone [usp Monograph]
100. (6alpha,11beta)-9-fluoro-11,17-dihydroxy-6-methylpregna-1,4-diene-3,20-dione
101. Ncgc00021575-03
102. Ncgc00021575-05
103. Ncgc00256631-01
104. As-12363
105. Fluorometholone Component Of Fml-s
106. Nci60_002886
107. Cs-0013955
108. Progesterone, 17-dihydroxy-6.alpha.-methyl-
109. D01367
110. 426f131
111. Q607349
112. Sr-01000003019
113. Sr-01000003019-2
114. Brd-k64862097-001-03-9
115. Brd-k64862097-001-12-0
116. Fluorometholone, British Pharmacopoeia (bp) Reference Standard
117. Fluorometholone, United States Pharmacopeia (usp) Reference Standard
118. Pregna-1,20-dione, 9-fluoro-11.beta.,17-dihydroxy-6.alpha.-methyl-
119. Progesterone, 1-dehydro-9-fluoro-11.beta., 17-dihydroxy-6.alpha.-methyl-
120. Pregna-1,20-dione, 9-fluoro-11,17-dihydroxy-6-methyl-, (6.alpha.,11.beta.)-
121. Pregna-1,4-diene-3,20-dione, 9-fluoro-11,17-dihydroxy-6-methyl-, (6i+/-,11i(2))-
122. (1r,2s,8s,10s,11s,14r,15s,17s)-14-acetyl-1-fluoro-14,17-dihydroxy-2,8,15-trimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-3,6-dien-5-one
123. (1r,2s,8s,10s,11s,14r,15s,17s)-14-acetyl-1-fluoro-14,17-dihydroxy-2,8,15-trimethyltetracyclo[8.7.0.02,7.011,15]heptadeca-3,6-dien-5-one
124. Pregna-1, 4-diene-3,20-dione, 9-fluoro-11,17-dihydroxy-6-methyl-, (6.alpha., 11.beta.)-
| Molecular Weight | 376.5 g/mol |
|---|---|
| Molecular Formula | C22H29FO4 |
| XLogP3 | 2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 1 |
| Exact Mass | 376.20498756 g/mol |
| Monoisotopic Mass | 376.20498756 g/mol |
| Topological Polar Surface Area | 74.6 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 787 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Fml |
| PubMed Health | Fluorometholone (Into the eye) |
| Drug Classes | Ophthalmologic Agent |
| Drug Label | FML (fluorometholone ophthalmic ointment) 0.1% is a sterile, topical anti-inflammatory agent for ophthalmic use.... |
| Active Ingredient | Fluorometholone |
| Dosage Form | Ointment; Suspension/drops |
| Route | Ophthalmic |
| Strength | 0.1% |
| Market Status | Prescription |
| Company | Allergan |
| 2 of 4 | |
|---|---|
| Drug Name | Fml forte |
| PubMed Health | Fluorometholone (Into the eye) |
| Drug Classes | Ophthalmologic Agent |
| Drug Label | FML FORTE (fluorometholone ophthalmic suspension, USP) 0.25% is a sterile, topical anti-inflammatory agent for ophthalmic use. Chemical NameFluorometholone: 9-Fluoro-11, 17-dihydroxy-6-methylpregna-1,4-diene-3,20-dione. Structural FormulaContai... |
| Active Ingredient | Fluorometholone |
| Dosage Form | Suspension/drops |
| Route | Ophthalmic |
| Strength | 0.25% |
| Market Status | Prescription |
| Company | Allergan |
| 3 of 4 | |
|---|---|
| Drug Name | Fml |
| PubMed Health | Fluorometholone (Into the eye) |
| Drug Classes | Ophthalmologic Agent |
| Drug Label | FML (fluorometholone ophthalmic ointment) 0.1% is a sterile, topical anti-inflammatory agent for ophthalmic use.... |
| Active Ingredient | Fluorometholone |
| Dosage Form | Ointment; Suspension/drops |
| Route | Ophthalmic |
| Strength | 0.1% |
| Market Status | Prescription |
| Company | Allergan |
| 4 of 4 | |
|---|---|
| Drug Name | Fml forte |
| PubMed Health | Fluorometholone (Into the eye) |
| Drug Classes | Ophthalmologic Agent |
| Drug Label | FML FORTE (fluorometholone ophthalmic suspension, USP) 0.25% is a sterile, topical anti-inflammatory agent for ophthalmic use. Chemical NameFluorometholone: 9-Fluoro-11, 17-dihydroxy-6-methylpregna-1,4-diene-3,20-dione. Structural FormulaContai... |
| Active Ingredient | Fluorometholone |
| Dosage Form | Suspension/drops |
| Route | Ophthalmic |
| Strength | 0.25% |
| Market Status | Prescription |
| Company | Allergan |
For the ophthalmic treatment of corticosteroid-responsive inflammation of the palpebral and bulbar conjunctiva, cornea and anterior segment of the globe.
FDA Label
Corticosteroids such as fluorometholone inhibit the inflammatory response to a variety of inciting agents and probably delay or slow healing. They inhibit the edema, fibrin deposition, capillary dilation, leukocyte migration, capillary proliferation, fibroblast proliferation, deposition of collagen, and scar formation associated with inflammation.
Anti-Inflammatory Agents
Substances that reduce or suppress INFLAMMATION. (See all compounds classified as Anti-Inflammatory Agents.)
Glucocorticoids
A group of CORTICOSTEROIDS that affect carbohydrate metabolism (GLUCONEOGENESIS, liver glycogen deposition, elevation of BLOOD SUGAR), inhibit ADRENOCORTICOTROPIC HORMONE secretion, and possess pronounced anti-inflammatory activity. They also play a role in fat and protein metabolism, maintenance of arterial blood pressure, alteration of the connective tissue response to injury, reduction in the number of circulating lymphocytes, and functioning of the central nervous system. (See all compounds classified as Glucocorticoids.)
Anti-Allergic Agents
Agents that are used to treat allergic reactions. Most of these drugs act by preventing the release of inflammatory mediators or inhibiting the actions of released mediators on their target cells. (From AMA Drug Evaluations Annual, 1994, p475) (See all compounds classified as Anti-Allergic Agents.)
S01BA07
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C05 - Vasoprotectives
C05A - Agents for treatment of hemorrhoids and anal fissures for topical use
C05AA - Corticosteroids
C05AA06 - Fluorometholone
D - Dermatologicals
D07 - Corticosteroids, dermatological preparations
D07A - Corticosteroids, plain
D07AB - Corticosteroids, moderately potent (group ii)
D07AB06 - Fluorometholone
D - Dermatologicals
D07 - Corticosteroids, dermatological preparations
D07X - Corticosteroids, other combinations
D07XB - Corticosteroids, moderately potent, other combinations
D07XB04 - Fluorometholone
D - Dermatologicals
D10 - Anti-acne preparations
D10A - Anti-acne preparations for topical use
D10AA - Corticosteroids, combinations for treatment of acne
D10AA01 - Fluorometholone
S - Sensory organs
S01 - Ophthalmologicals
S01B - Antiinflammatory agents
S01BA - Corticosteroids, plain
S01BA07 - Fluorometholone
S - Sensory organs
S01 - Ophthalmologicals
S01C - Antiinflammatory agents and antiinfectives in combination
S01CB - Corticosteroids/antiinfectives/mydriatics in combination
S01CB05 - Fluorometholone
There is no generally accepted explanation for the mechanism of action of ocular corticosteroids. However, corticosteroids are thought to act by the induction of phospholipase A2 inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor, arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A2. Their primary target is the cytosolic glucocorticoid receptor. After binding the receptor the newly formed receptor-ligand complex translocates itself into the cell nucleus, where it binds to many glucocorticoid response elements (GRE) in the promoter region of the target genes. The DNA bound receptor then interacts with basic transcription factors, causing the increase in expression of specific target genes.