







1. Cefmetazole Monosodium Salt
2. Cefmetazole Sodium
3. Cefmetazon
4. Cs 1170
5. Cs-1170
6. Cs1170
7. Monosodium Salt, Cefmetazole
8. Salt, Cefmetazole Monosodium
9. Sodium, Cefmetazole
10. U 72791a
11. U-72791a
12. U72791a
13. Zefazone
1. 56796-20-4
2. Cefmetazolo
3. Cefmetazolum
4. Cefmetazolum [inn-latin]
5. Cefmetazolo [inn-spanish]
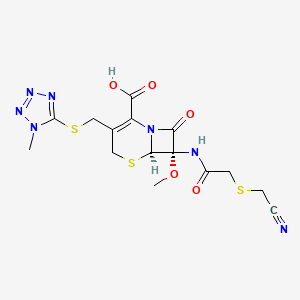
6. (6r,7s)-7-[[2-(cyanomethylsulfanyl)acetyl]amino]-7-methoxy-3-[(1-methyltetrazol-5-yl)sulfanylmethyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
7. Chebi:3489
8. U-72791
9. 3j962ujt8h
10. (6r,7s)-7-({[(cyanomethyl)sulfanyl]acetyl}amino)-7-methoxy-3-{[(1-methyl-1h-tetrazol-5-yl)sulfanyl]methyl}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
11. (6r,7s)-7-(2-((cyanomethyl)thio)acetamido)-7-methoxy-3-(((1-methyl-1h-tetrazol-5-yl)thio)methyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid
12. 56796-20-4 (free)
13. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-((((cyanomethyl)thio)acetyl)amino)-7-methoxy-3-(((1-methyl-1h-tetrazol-5-yl)thio)methyl)-8-oxo-, (6r-cis)-
14. 5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid,7-[[[(cyanomethyl)thio]acetyl]amino]-7-methoxy-3-[[(1-methyl-1h-tetrazol-5-yl)thio]methyl]-8-oxo-, (6r,7s)-
15. Cs 1170
16. Skf 83088
17. U 72791
18. Cefmetazole (usp/inn)
19. Einecs 260-384-2
20. Brn 0634647
21. Unii-3j962ujt8h
22. Cefmetazole [usan:usp:inn]
23. Cefmetazolesodium
24. Cefmetazole [mi]
25. Prestwick0_000700
26. Prestwick1_000700
27. Prestwick2_000700
28. Prestwick3_000700
29. Cefmetazole [inn]
30. Cefmetazole [usan]
31. Epitope Id:116204
32. Cefmetazole [vandf]
33. Cefmetazole [mart.]
34. Lopac0_000266
35. Bspbio_000859
36. Cefmetazole [usp-rs]
37. Cefmetazole [who-dd]
38. Schembl147832
39. Spbio_002780
40. Bpbio1_000945
41. Cefmetazole Sodium [jan]
42. Chembl1201195
43. Dtxsid7022756
44. Gtpl12215
45. Cefmetazole [usp Impurity]
46. Cefmetazole [usp Monograph]
47. Bcp11998
48. Hy-b1595
49. Zinc3830417
50. Bdbm50350471
51. Mfcd00865068
52. Akos015896219
53. Db00274
54. Sdccgsbi-0050254.p002
55. (6r,7s)-7-({[(cyanomethyl)thio]acetyl}amino)-7-(methyloxy)-3-{[(1-methyl-1h-tetrazol-5-yl)thio]methyl}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
56. (6r,7s)-7-(2-((cyanomethyl)thio)acetamido)-7-methoxy-3-(((1-methyl-1h-tetrazol-5-yl)thiomethyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, Sodium Salt
57. Ac-13154
58. Bs-42476
59. 2,6-difluoro-4-methoxyacetophenone
60. Cs-0013513
61. C08103
62. D00910
63. A831178
64. J-700159
65. Q5057238
66. (6r,7s)-7-({[(cyanomethyl)sulfanyl]acetyl}amino)-7-methoxy-3-{[(1-methyl-1h-tetrazol-5-yl)sulfanyl]methyl}-8-oxo-5-thia
67. (6r,7s)-7-(2-(cyanomethylthio)acetamido)-7-methoxy-3-((1-methyl-1h-tetrazol-5-ylthio)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
68. (6r,7s)-7-[[2-(cyanomethylthio)-1-oxoethyl]amino]-7-methoxy-3-[[(1-methyl-5-tetrazolyl)thio]methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
69. (6r,7s)-7-[2-(cyanomethylsulfanyl)ethanoylamino]-7-methoxy-3-[(1-methyl-1,2,3,4-tetrazol-5-yl)sulfanylmethyl]-8-oxidanylidene-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
70. (6r,7s)-7-{2-[(cyanomethyl)sulfanyl]acetamido}-7-methoxy-3-{[(1-methyl-1h-1,2,3,4-tetrazol-5-yl)sulfanyl]methyl}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
71. 7beta-{[(cyanomethyl)sulfanyl]acetamido}-7alpha-methoxy-3-{[(1-methyl-1h-tetrazol-5-yl)sulfanyl]methyl}ceph-3-em-4-carboxylic Acid
| Molecular Weight | 471.5 g/mol |
|---|---|
| Molecular Formula | C15H17N7O5S3 |
| XLogP3 | -0.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 9 |
| Exact Mass | 471.04533019 g/mol |
| Monoisotopic Mass | 471.04533019 g/mol |
| Topological Polar Surface Area | 239 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 818 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of infections caused by susceptible organisms.
Cefmetazole is a second-generation cephalosporin. The cephalosporins are bactericidal drugs with both gram-positive and gram-negative activity. They inhibit bacterial cell wall synthesis in a way similar to the penicillins. Cefmetazole is more active than 1st-generation cephalosporins against indole-positive Proteus, Serratia, anaerobic gram-negative bacilli (including B. fragilis), and some E. coli, Klebsiella, and P. mirabilis, but is less active than cefoxitin or cefotetan against most gram-negative bacilli.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01D - Other beta-lactam antibacterials
J01DC - Second-generation cephalosporins
J01DC09 - Cefmetazole
Absorption
Bioavailability is approximately 100% following intramuscular injection.
No appreciable metabolism.
1.50 ±0.14 hours
The bactericidal activity of cefmetazole results from the inhibition of cell wall synthesis via affinity for penicillin-binding proteins (PBPs).
