




1. Apacef
2. Apatef
3. Cefotan
4. Cefotetan Disodium
5. Cefotetan Disodium Salt
6. Ceftotan
7. Disodium Salt, Cefotetan
8. Disodium, Cefotetan
9. Ici 156834
10. Ici-156834
11. Ici156834
12. Salt, Cefotetan Disodium
13. Ym 09330
14. Ym-09330
15. Ym09330
1. 69712-56-7
2. Cefotetanum
3. Apacef
4. Cefotetanum [inn-latin]
5. Ici 156834
6. Ici-156834
7. Ici 156,834
8. 48spp0pa9q
9. Mls002153829
10. Chebi:3499
11. Ici-156,834
12. Ym 09330
13. Nsc-760045
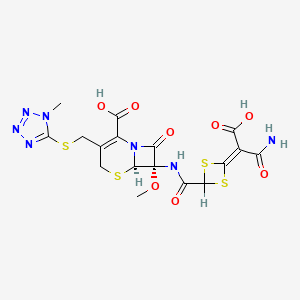
14. (6r,7s)-7-({[4-(2-amino-1-carboxy-2-oxoethylidene)-1,3-dithietan-2-yl]carbonyl}amino)-7-methoxy-3-{[(1-methyl-1h-tetrazol-5-yl)sulfanyl]methyl}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
15. Smr001233197
16. (6r,7s)-7-(4-(carbamoylcarboxymethylene)-1,3-dithiethane-2-carboxamido)-7-methoxy-3-(((1-methyl-1h-tetrazol-5- Yl)thio)methyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2- Carboxylic Acid
17. 7beta-({[4-(2-amino-1-carboxy-2-oxoethylidene)-1,3-dithietan-2-yl]carbonyl}amino)-7alpha-methoxy-3-{[(1-methyl-1h-tetrazol-5-yl)sulfanyl]methyl}-3,4-didehydrocepham-4-carboxylic Acid
18. Cefotetan Acid
19. (6r,7s)-7-[[4-(2-amino-1-carboxy-2-oxoethylidene)-1,3-dithietane-2-carbonyl]amino]-7-methoxy-3-[(1-methyltetrazol-5-yl)sulfanylmethyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
20. Unii-48spp0pa9q
21. Cefotetan [usan:usp:inn:ban]
22. Ncgc00016914-01
23. Einecs 274-093-3
24. Cas-69712-56-7
25. Ici156834
26. Brn 1208088
27. Cefotetan [inn]
28. Cefotetan [jan]
29. Cefotetan [mi]
30. Cefotetan [usan]
31. Prestwick0_000473
32. Prestwick1_000473
33. Prestwick2_000473
34. Prestwick3_000473
35. Cefotetan [vandf]
36. Dsstox_cid_2762
37. Cefotetan [mart.]
38. Cefotetan [who-dd]
39. Dsstox_rid_76720
40. Dsstox_gsid_22762
41. Schembl61376
42. Bspbio_000606
43. (6r,7s)-7-(4-(carbamoylcarboxymethylene)-1,3-dithiethane-2-carboxamido)-7-methoxy-3-(((1-methyl-1h-tetrazol-5-yl)thio)methyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid
44. Cid_53025
45. Spbio_002545
46. Cefotetan (jp17/usp/inn)
47. Bpbio1_000668
48. Chembl474579
49. Cefotetan [usp Impurity]
50. Dtxsid1022762
51. Bdbm80643
52. Gtpl10936
53. Cefotetan [usp Monograph]
54. Hms1569o08
55. Hms2096o08
56. Hms2234c15
57. Hms3713o08
58. Amy28799
59. Bcp10745
60. Hy-n6670
61. Zinc3830441
62. Tox21_110681
63. Mfcd00864983
64. Akos015896100
65. Ac-2141
66. Ccg-220473
67. Db01330
68. Nsc 760045
69. Ncgc00016914-06
70. Ncgc00179507-01
71. (6r,7s)-4-((2-carboxy-7-methoxy-3-(((1-methyl-1h-tetrazol-5-yl)thio)methyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-en-7-yl)carbamoyl)-1,3-dithietane-delta(sup 2,alpha)-malonamic Acid
72. (6r,7s)-7-[[[4-(2-amino-1-carboxy-2-oxoethylidene)-1,3-dithietan-2-yl]carbonyl]amino]-7-methoxy-3-[[(1-methyl-1h-tetrazol-5-yl)thio]methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
73. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-(((4-(2-amino-1-carboxy-2-oxoethylidene)-1,3-dithietan-2-yl)carbonyl)amino)-7-methoxy-3-(((1-methyl-1h-tetrazol-5-yl)thio)methyl)-8-oxo-, (6r-(6alpha,7alpha))-
74. As-56133
75. Ab00513847
76. Cs-0092723
77. C06886
78. D00260
79. Cefotetan, Antibiotic For Culture Media Use Only
80. 712c567
81. A836615
82. Sr-01000842155
83. Q2602246
84. Sr-01000842155-3
85. W-104601
86. (6r,7s)-4-((2-carboxy-7-methoxy-3-(((1-methyl-1h-tetrazol-5-yl)thio)methyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-en-7-yl)carbamoyl)-1,3-dithietane-.delta.(sup 2,.alpha.)-malonamic Acid
87. (6r,7s)-7-(4-(2-amino-1-carboxy-2-oxoethylidene)-1,3-dithietane-2-carboxamido)-7-methoxy-3-((1-methyl-1h-tetrazol-5-ylthio)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
88. (6r,7s)-7-[[[4-(2-amino-1-carboxy-2-oxoethylidene)-1,3-dithietan-2-yl]-oxomethyl]amino]-7-methoxy-3-[[(1-methyl-5-tetrazolyl)thio]methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
89. (6r,7s)-7-[[4-(1-amino-3-hydroxy-1,3-dioxopropan-2-ylidene)1,3-dithietane-2-carbonyl]amino]-7-methoxy-3-[(1-methyltetrazol-5-yl)sulfanylmethyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
90. (6r,7s)-7-[[4-(2-amino-1-carboxy-2-keto-ethylidene)-1,3-dithietane-2-carbonyl]amino]-8-keto-7-methoxy-3-[[(1-methyltetrazol-5-yl)thio]methyl]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
91. (6r,7s)-7-[[4-[1-azanyl-3-oxidanyl-1,3-bis(oxidanylidene)propan-2-ylidene]-1,3-dithietan-2-yl]carbonylamino]-7-methoxy-3-[(1-methyl-1,2,3,4-tetrazol-5-yl)sulfanylmethyl]-8-oxidanylidene-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
92. Disodium 7-[[4-(2-amino-1-carboxylato-2-oxo-ethylidene)-1,3-dithietane-2-carbonyl]amino]-7-methoxy-3-[(1-methyltetrazol-5-yl)sulfanylmethyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
| Molecular Weight | 575.6 g/mol |
|---|---|
| Molecular Formula | C17H17N7O8S4 |
| XLogP3 | 0.1 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 15 |
| Rotatable Bond Count | 9 |
| Exact Mass | 575.00214522 g/mol |
| Monoisotopic Mass | 575.00214522 g/mol |
| Topological Polar Surface Area | 321 Ų |
| Heavy Atom Count | 36 |
| Formal Charge | 0 |
| Complexity | 1090 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Cefotetan |
| PubMed Health | Cefotetan (Injection) |
| Drug Classes | Antibiotic |
| Drug Label | CEFOTAN (cefotetan disodium for injection) and CEFOTAN (cefotetan injection) in Galaxy * plastic container (PL 2040) as cefotetan disodium are sterile, semisynthetic, broad-spectrum, beta-lactamase resistant, cephalosporin (cephamycin) antibiotics... |
| Active Ingredient | Cefotetan disodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 2gm base/vial; eq 10gm base/vial; eq 1gm base/vial |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa; West-ward Pharm |
| 2 of 2 | |
|---|---|
| Drug Name | Cefotetan |
| PubMed Health | Cefotetan (Injection) |
| Drug Classes | Antibiotic |
| Drug Label | CEFOTAN (cefotetan disodium for injection) and CEFOTAN (cefotetan injection) in Galaxy * plastic container (PL 2040) as cefotetan disodium are sterile, semisynthetic, broad-spectrum, beta-lactamase resistant, cephalosporin (cephamycin) antibiotics... |
| Active Ingredient | Cefotetan disodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 2gm base/vial; eq 10gm base/vial; eq 1gm base/vial |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa; West-ward Pharm |
For prophylaxis and treatment of bacterial infections.
FDA Label
Cefotetan is a semisynthetic cephamycin antibiotic that is administered intravenously or intramuscularly. The drug is highly resistant to a broad spectrum of beta-lactamases and is active against a wide range of both aerobic and anaerobic gram-positive and gram-negative microorganisms.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01D - Other beta-lactam antibacterials
J01DC - Second-generation cephalosporins
J01DC05 - Cefotetan
Route of Elimination
No active metabolites of cefotetan have been detected; however, small amounts (less than 7%) of cefotetan in plasma and urine may be converted to its tautomer, which has antimicrobial activity similar to the parent drug. In normal patients, from 51% to 81% of an administered dose of Cefotetan is excreted unchanged by the kidneys over a 24 hour period, which results in high and prolonged urinary concentrations.
Volume of Distribution
10.4 L [elderly patients (greater than 65 years) with normal renal function]
10.3 L [healthy volunteers (aged 25 to 28 years)]
Clearance
1.8 +/- 0.1 L/h [elderly patients with normal renal function (.65 years)]
1.8 +/- 0.3 L/h [healthy volunteers (aged 25 to 28 years)]
No active metabolites of cefotetan have been detected; however, small amounts (less than 7%) of cefotetan in plasma and urine may be converted to its tautomer, which has antimicrobial activity similar to the parent drug.
In volunteers with reduced renal function, the plasma half-life of cefotetan is prolonged
The bactericidal action of cefotetan results from inhibition of cell wall synthesis by binding and inhibiting the bacterial penicillin binding proteins which help in the cell wall biosynthesis.