






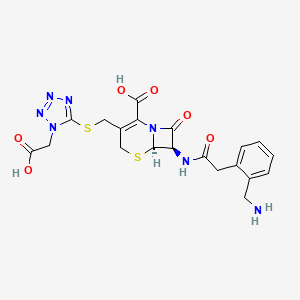

1. 7-(alpha-(2-aminomethylphenyl)acetamido)-3-((1-carboxymethyltetrazol-5-ylthio)methyl)-3-cephem-4-carboxylic Acid
2. Bl-s 786
3. Bl-s786r
4. Cefaronide
5. Ceforanide, Monosodium Salt
1. 60925-61-3
2. Precef
3. Ceforanido
4. Ceforanidum
5. Ceforanidum [inn-latin]
6. Ceforanido [inn-spanish]
7. Bl-s786
8. (6r,7r)-7-[[2-[2-(aminomethyl)phenyl]acetyl]amino]-3-[[1-(carboxymethyl)tetrazol-5-yl]sulfanylmethyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
9. Chebi:3495
10. 7-(o-(aminomethyl)phenylacetamido)-3-(((1-(carboxymethyl)-1h-tetrazol-5-yl)thio)methyl)-3-cephem-4-carboxylic Acid
11. 8m1yf8951v
12. Nsc-760049
13. (6r,7r)-7-{2-[2-(aminomethyl)phenyl]acetamido}-3-({[1-(carboxymethyl)-1h-1,2,3,4-tetrazol-5-yl]sulfanyl}methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
14. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-(((2-(aminomethyl)phenyl)acetyl)amino)-3-(((1-(carboxymethyl)-1h-tetrazol-5-yl)thio)methyl)-8-oxo-, (6r-trans)-
15. 7-[o-(aminomethyl)phenylacetamido]-3-[[[1-(carboxymethyl)-1h-tetrazol-5-yl]thio]methyl]-3-cephem-4-carboxylic Acid
16. 7beta-[2-(aminomethyl)phenyl]acetamido-3-{[1-(carboxymethyl)-1h-tetrazol-5-yl]sulfanyl}methyl-3,4-didehydrocepham-4-carboxylic Acid
17. Bl-s 786
18. Precef (tn)
19. Ceforanide (usp/inn)
20. Sr-01000872614
21. Unii-8m1yf8951v
22. Ceforanide [usan:usp:inn:ban]
23. Ncgc00016897-01
24. Cas-60925-61-3
25. (6r,7r)-7-(2-(alpha-amino-o-tolyl)acetamido)-3-(((1-(carboxymethyl)-1h-tetrazol-5-yl)thio)methyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid
26. Ceforanide [mi]
27. Ceforanide [inn]
28. Prestwick0_000470
29. Prestwick1_000470
30. Prestwick2_000470
31. Prestwick3_000470
32. Ceforanide [usan]
33. Dsstox_cid_2760
34. Ceforanide [vandf]
35. Ceforanide [mart.]
36. Ceforanide(200mg)
37. Ceforanide [usp-rs]
38. Ceforanide [who-dd]
39. Dsstox_rid_76719
40. Dsstox_gsid_22760
41. Bspbio_000580
42. (6r-trans)-7-(((2-(aminomethyl)phenyl)acetyl)amino)-3-(((1-(carboxymethyl)-1h-tetrazol-5-yl)thio)methyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid
43. Schembl122072
44. Spbio_002519
45. Bpbio1_000638
46. Ceforanide [orange Book]
47. Chembl1201046
48. Dtxsid1022760
49. Gtpl12218
50. Ceforanide [usp Impurity]
51. Hms1569m22
52. Hms2096m22
53. Hms3713m22
54. (6r,7r)-7-(2-(2-(aminomethyl)phenyl)acetamido)-3-(((1-(carboxymethyl)-1h-tetrazol-5-yl)thio)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
55. Hy-b1297
56. Zinc3830434
57. Tox21_110670
58. Ccg-220470
59. Db00923
60. Nsc 760049
61. Ncgc00179514-05
62. (6r,7r)-7-({[2-(aminomethyl)phenyl]acetyl}amino)-3-({[1-(carboxymethyl)-1h-tetrazol-5-yl]sulfanyl}methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
63. (6r,7r)-7-({[2-(aminomethyl)phenyl]acetyl}amino)-3-({[1-(carboxymethyl)-1h-tetrazol-5-yl]thio}methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
64. As-15783
65. Ab00513845
66. Cs-0013066
67. S5081
68. C06884
69. D00259
70. D81832
71. 925c613
72. A913663
73. Q5057287
74. Sr-01000872614-2
75. Sr-01000872614-3
76. Brd-k37848908-001-03-1
77. (6r,7r)-7-(2-(.alpha.-amino-o-tolyl)acetamido)-3-(((1-(carboxymethyl)-1h-tetrazol-5-yl)thio)methyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid
78. (6r,7r)-7-(2-(2-(aminomethyl)phenyl)acetamido)-3-(((1-(carboxymethyl)-1h-tetrazol-5-yl)thio)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylicacid
79. 7-[[2-[2-(aminomethyl)phenyl]acetyl]amino]-3-[[1-(carboxymethyl)tetrazol-5-yl]sulfanylmethyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
| Molecular Weight | 519.6 g/mol |
|---|---|
| Molecular Formula | C20H21N7O6S2 |
| XLogP3 | -3.2 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 10 |
| Exact Mass | 519.09947376 g/mol |
| Monoisotopic Mass | 519.09947376 g/mol |
| Topological Polar Surface Area | 244 Ų |
| Heavy Atom Count | 35 |
| Formal Charge | 0 |
| Complexity | 905 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of infections caused by susceptible organisms.
Ceforanide is a semisynthetic second-generation cephalosporin. The cephalosporins are bactericidal drugs with both gram-positive and gram-negative activity. They inhibit bacterial cell wall synthesis in a way similar to the penicillins.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01D - Other beta-lactam antibacterials
J01DC - Second-generation cephalosporins
J01DC11 - Ceforanide
Absorption
Rapidly absorbed following intramuscular injection.
The major drug elimination route was urinary excretion with 85% of the dose being excreted unchanged in the urine within 12 hr, and no metabolites with antibiotic activity were observed in urine.
2.6 to 2.98 hours
The bactericidal activity of ceforanide results from the inhibition of cell wall synthesis via affinity for penicillin-binding proteins (PBPs).
