







1. 886-74-8
2. Maolate
3. Rinlaxer
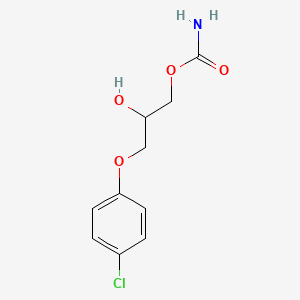
4. 3-(4-chlorophenoxy)-2-hydroxypropyl Carbamate
5. 3-(p-chlorophenoxy)-1,2-propanediol 1-carbamate
6. 3-(p-chlorophenoxy)-2-hydroxypropyl Carbamate
7. Chlorphensin Carbamate
8. Chlorphenesincarbamate
9. 3-(4-chlorophenoxy)-1,2-propanediol-1-carbamate
10. U-19,646
11. 1,2-propanediol, 3-(4-chlorophenoxy)-, 1-carbamate
12. Nsc 82943
13. Carbamic Acid, 3-(p-chlorophenoxy)-2-hydroxypropyl Ester
14. [3-(4-chlorophenoxy)-2-hydroxypropyl] Carbamate
15. 3-(p-chlorophenoxy)-1,2-propanediol-1-carbamate
16. U 19646
17. 1,2-propanediol, 3-(p-chlorophenoxy)-, 1-carbamate
18. Nsc-82943
19. Hqc4wi89yg
20. 1,2-propanediol,3-(4-chlorophenoxy)-, 1-carbamate
21. Carbamic Acid 3-(p-chlorophenoxy)-2-hydroxypropyl Ester
22. 57u5yi11wp
23. Chebi:3643
24. Chlorphenesin Carbamate, (-)-
25. 1,2-propanediol-3-(p-chlorophenoxy)-1-carbamate
26. Nsc82943
27. Kolpicortin-sine
28. (3-p-chlorophenoxy)-2-hydroxypropylcarbamate
29. U-19646
30. 1,2-propanediol, 3-(4-chlorophenoxy)-, 1-carbamate, (-)-
31. Maolate; Nsc 82943; Rhnesicn; Rinlaxer; U 19646; U-19,646
32. 126632-50-6
33. Hsdb 3031
34. Chlorphenesin Carbamate [usan:jan]
35. Einecs 212-954-7
36. Brn 1978575
37. Unii-57u5yi11wp
38. Ncgc00016548-01
39. Cas-886-74-8
40. Prestwick_882
41. Maolate (tn)
42. Prestwick0_000234
43. Prestwick1_000234
44. Prestwick2_000234
45. Prestwick3_000234
46. Unii-hqc4wi89yg
47. Dsstox_cid_2803
48. Wln: Zvo1yq1or Dg
49. Dsstox_rid_76735
50. Dsstox_gsid_22803
51. Schembl34492
52. Bspbio_000307
53. Mls002154243
54. Spbio_002228
55. Bpbio1_000339
56. Chembl607710
57. G014xc07gh
58. Dtxsid5022803
59. Glxc-26272
60. Hms1568p09
61. Hms2095p09
62. Hms2232o23
63. Hms3371a08
64. Hms3712p09
65. Chlorphenesin Carbamate [mi]
66. Tox21_110487
67. Chlorphenesin Carbamate [jan]
68. Chlorphenesin Carbamate [hsdb]
69. Chlorphenesin Carbamate [usan]
70. Chlorphenesin Carbamate (jp17/usan)
71. Ccg-220234
72. Chlorphenesin Carbamate [mart.]
73. Chlorphenesin Carbamate [vandf]
74. Chlorphenesin Carbamate, (+)-
75. Db14656
76. Chlorphenesin Carbamate [who-dd]
77. 1, 3-(p-chlorophenoxy)-, 1-carbamate
78. Ncgc00179607-01
79. Ncgc00179607-03
80. 1, 3-(4-chlorophenoxy)-, 1-carbamate
81. Smr001233511
82. Chlorphenesin Carbamate [green Book]
83. Db-057097
84. Hy-107944
85. Chlorphenesin Carbamate [orange Book]
86. Cs-0030953
87. Ft-0602972
88. C07930
89. D00770
90. [3-(4-chlorophenoxy)-2-hydroxy-propyl] Carbamate
91. Sr-01000841824
92. 3-[(4-chlorophenyl)oxy]-2-hydroxypropyl Carbamate
93. Sr-01000841824-2
94. W-100393
95. Brd-a39230911-001-03-7
96. Q27106156
97. 1,2-propanediol, 3-(4-chlorophenoxy)-, 1-carbamate, (+)-
| Molecular Weight | 245.66 g/mol |
|---|---|
| Molecular Formula | C10H12ClNO4 |
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 6 |
| Exact Mass | 245.0454856 g/mol |
| Monoisotopic Mass | 245.0454856 g/mol |
| Topological Polar Surface Area | 81.8 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 219 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
IT IS EMPLOYED TO DIMINISH SKELETAL MUSCLE SPASMS RESULTING FROM TRAUMA , INFLAMMATION, VERTEBRAL DISK SYNDROME, OSTEOARTHRITIS, AND RHEUMATOID ARTHRITIS.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 860
APPLICATION: CENTRALLY ACTIVE SKELETAL MUSCLE RELAXANT SIMILAR IN ITS ACTIONS TO METHOCARBAMOL...
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 860
IT MAY BE USEFUL AS TEMPORARY ADJUNCT TO PHYSIOTHERAPY & OTHER APPROPRIATE MEASURES IN THE TREATMENT OF THE DISCOMFORT PRODUCED BY SKELETAL MUSCLE SPASM OF LOCAL ORIGIN.
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 1026
MEDICATION (VET): /USED/ AS AN ADJUNCT TO OTHER THERAPY FOR ACUTE INFLAMMATORY OR TRAUMATIC SKELETAL MUSCLE INVOLVEMENT INCLUDING SPRAINS & INTERVERTEBRAL DISC SYNDROMES.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 108
THESE DRUGS MAY TEMPORARILY ABATE SOME OF THE SYMPTOMS OF CEREBRAL PALSY, BUT THEY HAVE MINOR ROLE IN OVERALL MGMNT OF THIS DISEASE... ALL CENTRALLY ACTING MUSCLE RELAXANTS PRODUCE SOME SEDATION, AT LEAST AT HIGHEST DOSES EMPLOYED CLINICALLY. /MUSCLE RELAXANTS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 241
PERSONS TAKING DRUG SHOULD NOT DRIVE, OPERATE MACHINERY, OR UNDERTAKE ACTIVITIES THAT REQUIRE ALERTNESS, JUDGMENT, OR MENTATION.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 860
...PROBABLY HAS AN ADDICTION LIABILITY, AS DO OTHER CARBAMATES.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 860
VET: DO NOT ADMIN TO ANY ANIMAL WITH SIGNIFICANT LIVER PATHOLOGY. LIVER FUNCTION MAY BE ADVERSELY AFFECTED DURING ITS USE.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 108
ORAL DOSES ARE READILY ABSORBED /IN ANIMALS/.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 108
MUCH OF DOSE OF CHLORPHENESIN CARBAMATE...IS RAPIDLY EXCRETED IN URINE OF RATS & OF MAN.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 1: A Review of the Literature Published Between 1960 and 1969. London: The Chemical Society, 1970., p. 175
AFTER ORAL ADMIN OF (14)C-LABELED CHLORPHENESIN CARBAMATE TO RATS, IT WAS ABSORBED FROM GI TRACT; EXCRETED IN URINE ABOUT 90%; 36% IN BILE. RADIOACTIVITY WAS DISTRIBUTED IN ALMOST ALL TISSUES, HIGHEST IN LIVER, BRAIN & SPINAL CORD.
NOZU T ET AL; OYO YAKURI 14(1) 1 (1977)
MAJOR METABOLITE (49% OF DOSE) IS ETHER GLUCURONIDE OF CHLORPHENESIN CARBAMATE...
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 1: A Review of the Literature Published Between 1960 and 1969. London: The Chemical Society, 1970., p. 175
5% APPEARED IN URINE AS P-CHLOROPHENOXYLACTIC ACID, 17% AS P-CHLOROPHENOXYACETIC ACID, AND 9% AS SULFATE CONJUGATE OF P-CHLOROPHENOL...
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 1: A Review of the Literature Published Between 1960 and 1969. London: The Chemical Society, 1970., p. 175
MAJOR METABOLITES IN A 48-HR URINE SAMPLE FROM RATS GIVEN (14)C-LABELED CHLORPHENESIN CARBAMATE WERE GLUCURONIDE, P-CHLOROPHENOXYLACTIC ACID, P-CHLOROPHENOXYACETIC ACID, & P-CHLOROPHENOL.
NOZU T ET AL; OYO YAKURI 14(1) 1 (1977)
MUSCLE RELAXANTS CAUSE SKELETAL MUSCULAR RELAXATION, WITHOUT LOSS OF CONSCIOUSNESS, AS RESULT OF SELECTIVE ACTION UPON CNS. ... PROMINENT EFFECT OF MUSCLE RELAXANTS IS TO DEPRESS SPINAL POLYSYNAPTIC REFLEXES PREFERENTIALLY OVER MONOSYNAPTIC REFLEXES. ... MECHANISM OF ACTION...REMAIN TO BE ELUCIDATED. /MUSCLE RELAXANTS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 239
