



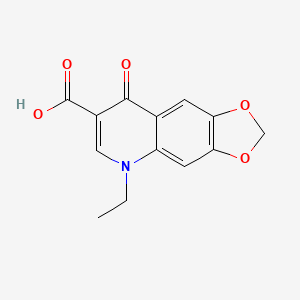

1. Acid, Oxolinic
2. Gramurin
3. Oxolinate, Sodium
4. Sodium Oxolinate
1. 14698-29-4
2. Nidantin
3. Dioxacin
4. Emyrenil
5. Utibid
6. Prodoxal
7. Prodoxol
8. Gramurin
9. Oksaren
10. Oxolinic
11. Ossian
12. Uroxol
13. Acide Oxolinique
14. Uritrate
15. Urotrate
16. Oxoboi
17. Pietil
18. Starner
19. Ultibid
20. Acido Oxolinico
21. Uro-alvar
22. Acidum Oxolinicum
23. Nsc-110364
24. Urinox
25. 5-ethyl-8-oxo-5,8-dihydro-[1,3]dioxolo[4,5-g]quinoline-7-carboxylic Acid
26. W 4565
27. 5-ethyl-8-oxo-5,8-dihydro[1,3]dioxolo[4,5-g]quinoline-7-carboxylic Acid
28. 5-ethyl-8-oxo-[1,3]dioxolo[4,5-g]quinoline-7-carboxylic Acid
29. Aqualinic
30. Nsc 110364
31. 1-ethyl-6,7-methylenedioxy-4-quinolone-3-carboxylic Acid
32. Mfcd00056775
33. 5-ethyl-5,8-dihydro-8-oxo-1,3-dioxolo(4,5-g)quinoline-7-carboxylic Acid
34. 1-ethyl-1,4-dihydro-6,7-methylenedioxy-4-oxo-3-quinolinecarboxylic Acid
35. Oxolinic Acid Impurity B
36. 1,3-dioxolo(4,5-g)quinoline-7-carboxylic Acid, 5-ethyl-5,8-dihydro-8-oxo-
37. L0a22b22ft
38. Chebi:138856
39. 1,3-dioxolo[4,5-g]quinoline-7-carboxylic Acid, 5-ethyl-5,8-dihydro-8-oxo-
40. Nsc110364
41. Ncgc00015762-06
42. Cistopax
43. Orthurine
44. Tiurasin
45. Acido Ossolico
46. Cas-14698-29-4
47. Dsstox_cid_1089
48. W-4565
49. Dsstox_rid_75935
50. Dsstox_gsid_21089
51. 16172-03-5
52. Acido Ossolico [dcit]
53. 5-ethyl-5,8-dihydro-8-oxo-1,3-dioxolo[4,5-g]quinoline-7-carboxylic Acid
54. Acide Oxolinique [inn-french]
55. Acido Oxolinico [inn-spanish]
56. Acidum Oxolinicum [inn-latin]
57. Ccris 6301
58. Hsdb 3243
59. Sr-01000076042
60. Einecs 238-750-8
61. Brn 0620635
62. Unii-l0a22b22ft
63. Inoxyl
64. Oxolinic-acid
65. Oxolinic Acid [usan:inn:ban]
66. S-0208
67. Aqualinic (tn)
68. Quinolone Antibiotic
69. Prestwick_629
70. Spectrum_001397
71. Prestwick0_000193
72. Prestwick1_000193
73. Prestwick2_000193
74. Prestwick3_000193
75. Spectrum2_000933
76. Spectrum3_001490
77. Spectrum4_000073
78. Spectrum5_001176
79. Lopac-o-0877
80. 5,8-dihydro-5-ethyl-8-oxo-1,3-dioxolo[4,5-g]quinoline-7-carboxylic Acid
81. Oxolinic Acid [mi]
82. Oxolinic Acid (usan/inn)
83. Oxolinic Acid [inn]
84. Lopac0_000952
85. Oprea1_169598
86. Schembl24445
87. Bspbio_000145
88. Bspbio_003079
89. Kbiogr_000625
90. Kbioss_001877
91. Oxolinic Acid [hsdb]
92. Oxolinic Acid [usan]
93. Mls000028501
94. Divk1c_000659
95. Spectrum1502030
96. Spbio_000866
97. Spbio_002066
98. Oxolinic Acid [mart.]
99. Bpbio1_000161
100. Chembl416755
101. Zinc1875
102. Oxolinic Acid [who-dd]
103. Dtxsid1021089
104. Hms502a21
105. Kbio1_000659
106. Kbio2_001877
107. Kbio2_004445
108. Kbio2_007013
109. Kbio3_002579
110. Ninds_000659
111. Hms1568h07
112. Hms1921f12
113. Hms2092p09
114. Hms2095h07
115. Hms3262p06
116. Hms3712h07
117. Oxolinic Acid, Analytical Standard
118. Pharmakon1600-01502030
119. Oxolinic Acid, Quinolone Antibiotic
120. Act03284
121. Hy-b1002
122. Tox21_110216
123. Tox21_202787
124. Tox21_500952
125. Ac8065
126. Bbl009934
127. Ccg-39666
128. Nsc758177
129. Oxolinic Acid [ep Monograph]
130. S4537
131. Stk801351
132. 5-ethyl-8-oxo-5-hydro-2h-1,3-dioxoleno[4,5-g]quinoline-7-carboxylic Acid
133. Akos000282622
134. Tox21_110216_1
135. Cs-4499
136. Db13627
137. Lp00952
138. Nsc-758177
139. Sdccgsbi-0050926.p004
140. Idi1_000659
141. Ncgc00015762-01
142. Ncgc00015762-02
143. Ncgc00015762-03
144. Ncgc00015762-04
145. Ncgc00015762-05
146. Ncgc00015762-07
147. Ncgc00015762-08
148. Ncgc00015762-09
149. Ncgc00015762-10
150. Ncgc00015762-11
151. Ncgc00015762-13
152. Ncgc00015762-17
153. Ncgc00093361-02
154. Ncgc00093361-03
155. Ncgc00093361-04
156. Ncgc00093361-05
157. Ncgc00260333-01
158. Ncgc00261637-01
159. As-12087
160. Smr000058314
161. Sy066716
162. Sbi-0050926.p003
163. Db-042865
164. Eu-0100952
165. Ft-0637128
166. Oxolinic Acid 100 Microg/ml In Acetonitrile
167. Vu0243182-3
168. D02301
169. O 0877
170. Wln: T C566 Do Fo Jn Mv Ehj J2 Lvq
171. 698o294
172. A808577
173. Q287840
174. Sr-01000076042-1
175. Sr-01000076042-4
176. Sr-01000076042-6
177. W-108119
178. Brd-k73394555-001-08-6
179. 5-ethyl-5,3-dioxolo[4,5-g]quinoline-7-carboxylic Acid
180. F3351-0487
181. 1-ethyl-1,7-methylenedioxy-4-oxo-3-quinolinecarboxylic Acid
182. Oxolinic Acid, European Pharmacopoeia (ep) Reference Standard
183. 1,5-g]quinoline-7-carboxylic Acid, 5-ethyl-5,8-dihydro-8-oxo-
184. 1.4-dihydro-1-ethyl-6,7-methylenedioxy-4-oxoquinoline-3-carboxylic Acid
185. 5-ethy1-5,8-dihydro-8-oxo-1,3-dioxolo[4,5]quinoline-7-carboxylic Acid
186. 5-ethyl-5,8-dihydro-8-oxo(1,3)dioxolo(4,5-g)quinoline-7-carboxylic Acid
187. 5-ethyl-8-oxo-2h,5h,8h-[1,3]dioxolo[4,5-g]quinoline-7-carboxylic Acid
188. 5-ethyl-8-oxo-5,8-dihydro[1,3]dioxolo[4,5-g]quinoline-7-carboxylic Acid #
189. W-4565; 5,8-dihydro-5-ethyl-8-oxo-1,3-dioxolo[4,5-g]quinoline-7-carboxylic Acid
| Molecular Weight | 261.23 g/mol |
|---|---|
| Molecular Formula | C13H11NO5 |
| XLogP3 | -0.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 2 |
| Exact Mass | 261.06372245 g/mol |
| Monoisotopic Mass | 261.06372245 g/mol |
| Topological Polar Surface Area | 76.1 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 446 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Infective Agents, Quinolone; Anti-Infective Agents, Urinary
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
DRUG HAS CHEMICAL STRUCTURE, MECHANISM OF ACTION, SPECTRUM OF ACTIVITY & POTENTIAL FOR TOXICITY THAT RESEMBLES NALIDIXIC ACID.
Miller, R. R., and D. J. Greenblatt. Handbook of Drug Therapy. New York: Elsevier North Holland, 1979., p. 182
OXOLINIC ACID IS ALTERNATIVE FORM OF THERAPY FOR PENICILLIN-SENSITIVE OR CEPHALOSPORIN-SENSITIVE ADULT WHO HAS RECURRENT URINARY TRACT INFECTION CAUSED BY SUSCEPTIBLE ESCHERICHIA COLI OR PROTEUS MIRABILIS THAT IS NOT COMPLICATED BY BACTEREMIA.
Miller, R. R., and D. J. Greenblatt. Handbook of Drug Therapy. New York: Elsevier North Holland, 1979., p. 183
...EXERTS IN VITRO ACTIVITY AGAINST MOST GRAM-NEGATIVE AEROBIC BACILLI THAT CAUSE BACTERIAL URINARY TRACT INFECTIONS. MAJORITY OF ESCHERICHIA COLI, KLEBSIELLA SP, ENTEROBACTER SP & PROTEUS SP ARE SUSCEPTIBLE. ... SALMONELLA, SHIGELLA & NEISSERIA (MENINGITIDIS, GONORRHEAE) ARE SUSCEPTIBLE...STAPHYLOCOCCUS AUREUS...
Miller, R. R., and D. J. Greenblatt. Handbook of Drug Therapy. New York: Elsevier North Holland, 1979., p. 182
For more Therapeutic Uses (Complete) data for OXOLINIC ACID (6 total), please visit the HSDB record page.
PSEUDOMONAS AERUGINOSA & ACINETOBACTER CALCOACETICUS (VAR LIVOFFI & VAR ANITRATUS) ARE UNIFORMLY RESISTANT...
Miller, R. R., and D. J. Greenblatt. Handbook of Drug Therapy. New York: Elsevier North Holland, 1979., p. 182
WHEN BACTERIAL RESISTANCE DEVELOPS...IT IS USUALLY RAPID. THEREFORE, IF FOLLOW-UP CULTURES INDICATE THAT URINE IS NOT STERILE WITHIN 48-72 HR, TREATMENT CAN PROBABLY BE CONSIDERED FAILURE SINCE ORGANISM BY THEN WILL HAVE DEVELOPED RESISTANCE. ... CROSS RESISTANCE TO NALIDIXIC ACID HAS BEEN REPORTED.
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 794
...HAS STIMULATORY EFFECT ON CNS, & SHOULD NOT BE PRESCRIBED FOR PT WITH KNOWN SEIZURE DISORDERS.
Miller, R. R., and D. J. Greenblatt. Handbook of Drug Therapy. New York: Elsevier North Holland, 1979., p. 184
...HAS FAIRLY NARROW ANTIBACTERIAL SPECTRUM &, THEREFORE, BACTERIAL CULTURE & SENSITIVITY TESTS GENERALLY SHOULD BE PERFORMED PRIOR TO ITS USE.
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 794
For more Drug Warnings (Complete) data for OXOLINIC ACID (7 total), please visit the HSDB record page.
Topoisomerase II Inhibitors
Compounds that inhibit the activity of DNA TOPOISOMERASE II. Included in this category are a variety of ANTINEOPLASTIC AGENTS which target the eukaryotic form of topoisomerase II and ANTIBACTERIAL AGENTS which target the prokaryotic form of topoisomerase II. (See all compounds classified as Topoisomerase II Inhibitors.)
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Anti-Infective Agents, Urinary
Substances capable of killing agents causing urinary tract infections or of preventing them from spreading. (See all compounds classified as Anti-Infective Agents, Urinary.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01M - Quinolone antibacterials
J01MB - Other quinolones
J01MB05 - Oxolinic acid
AFTER INGESTION OF OXOLINIC ACID, 5-ETHYL-5,8-DIHYDRO-8 -OXO-1,3-DIOXOLO-[4,5-G]QUINOLINE-7-CARBOXYLIC ACID, BY HUMAN SUBJECTS, URINE CONTAINED SMALL QUANTITY OF BIOLOGICALLY-INACTIVE & UNIDENTIFIED OXOLINIC ACID COMPLEX, BUT NO UNCHANGED OXOLINIC ACID.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 1: A Review of the Literature Published Between 1960 and 1969. London: The Chemical Society, 1970., p. 206
OF ORAL DOSE OF ANTIBACTERIAL, (14)C-OXOLINIC ACID, THERE WAS EXCRETED IN 24-HR URINE & FECES RESPECTIVELY, 27 & 41% BY RAT, 19 & 14% BY DOG, 49 & 37% BY RABBIT, & 35 & 10% BY MAN. BLOOD LEVELS OF (14)C PEAKED AFTER 4 HR IN DOG & MAN, & AFTER 6 HR IN RAT & RABBIT.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 111
AFTER ORAL ADMIN...RAPIDLY ABSORBED FROM GI TRACT. PEAK SERUM CONCN OF BIOLOGICALLY ACTIVE UNCONJUGATED DRUG ARE ATTAINED IN 2-4 HR & RANGE FROM 1.8-3.6 UG/ML. LOWER SERUM LEVELS...OCCUR DURING 1ST 3 DAYS OF DOSING, SUGGESTING SLOW DISTRIBUTION... PROTEIN BINDING OF DRUG IS ABOUT 77-81%.
Miller, R. R., and D. J. Greenblatt. Handbook of Drug Therapy. New York: Elsevier North Holland, 1979., p. 183
750 MG ADMIN TWICE/DAY FOR 7 DAYS STUDIED IN 10 HEALTHY WOMEN. WHEN TAKEN WITH FOOD, EXCRETION RETARDED BY 6 HR BUT 48 HR RECOVERY NOT DECR.
MANNISTO PT; CLIN PHARMACOL THER 19(JAN) 37-46 (1976)
For more Absorption, Distribution and Excretion (Complete) data for OXOLINIC ACID (6 total), please visit the HSDB record page.
YIELDS 1-ETHYL-1,4-DIHYDRO-7-HYDROXY-6-METHOXY-6-OXOQUINOLINE-3-CARBOXYLIC ACID IN RAT, RABBIT, DOG; YIELDS OXOLINOYL-BETA-D-GLUCURONIC ACID IN RAT, RABBIT, DOG; CREW, MC, MELGAR, MD, HAYNES, LJ, GALA, RL, & DICARLO, FJ, XENOBIOTICA, 1, 193 (1971). /FROM TABLE/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. 11
MAJOR URINARY METABOLITE WAS GLUCURONIDE OF OXOLINIC ACID...THIS COMPD WAS BIOLOGICALLY ACTIVE WHEREAS ALMOST ALL DRUG GLUCURONIDES ARE BIOLOGICALLY INERT...
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 1: A Review of the Literature Published Between 1960 and 1969. London: The Chemical Society, 1970., p. 206
AFTER REPEATED DOSING, BIPHASIC EXCRETION PATTERN HAS BEEN OBSERVED. INITIAL PHASE IS RAPID WITH T/2 OF ABOUT 1.5 HR, & IS FOLLOWED BY SLOW PHASE WITH T/2 OF ABOUT 15 HR. URINARY CONCN OF UNCONJUGATED DRUG RANGE FROM 15-155 UG/ML IN PT WITH NORMAL RENAL FUNCTION.
Miller, R. R., and D. J. Greenblatt. Handbook of Drug Therapy. New York: Elsevier North Holland, 1979., p. 183
...INTERFERENCE WITH DEOXYRIBONUCLEIC ACID (DNA) SYNTH. OXOLINIC ACID, HOWEVER, HAS DEMONSTRATED 10-FOLD GREATER /THAN NALIDIXIC ACID/ ABILITY TO INHIBIT DNA REPLICATION, WHICH IS PARALLELED BY ITS GREATER IN VITRO ACTIVITY FOR ENTEROBACTERIACEAE.
Miller, R. R., and D. J. Greenblatt. Handbook of Drug Therapy. New York: Elsevier North Holland, 1979., p. 182