
1. Pred Forte
2. Prednisolone 21-acetate
3. Scherisolone-kristall Suspension
1. 52-21-1
2. Prednisolone 21-acetate
3. Omnipred
4. Econopred
5. Pricortin
6. Cormalone
7. Cortipred
8. Deltilen
9. Durapred
10. Predicort
11. Prednidoren
12. Supercortyl
13. Prenema
14. Meticortelone Acetate
15. Prednelan-n
16. Delcort-e
17. Pred Mild
18. Pred Forte
19. Flo-pred
20. Prediacortine
21. Nisolone
22. Prednisolone-21-acetate
23. Predalone 50
24. Predonine Injection
25. Nsc-10966
26. Mls000028512
27. Chebi:8380
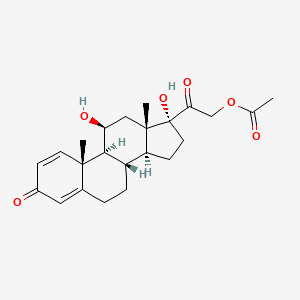
28. [2-[(8s,9s,10r,11s,13s,14s,17r)-11,17-dihydroxy-10,13-dimethyl-3-oxo-7,8,9,11,12,14,15,16-octahydro-6h-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl] Acetate
29. Prednisolone (21-acetate)
30. Smr000058326
31. Econopred Plus
32. 8b2807733d
33. Nsc 10966
34. Dsstox_cid_3502
35. Dsstox_rid_77056
36. Dsstox_gsid_23502
37. Prednisoloneacetate
38. 21-acetoxy-1,4-pregnadiene-11beta,17alpha-diol-3,20-dione
39. Pred-forte
40. Cetapred Ointment
41. Pred-g Liquifilm
42. Vasocidin Ointment
43. Cas-52-21-1
44. Blephamide Liquifilm
45. Einecs 200-134-1
46. Prednisolone Acetate (omnipred)
47. Brn 3111798
48. Prednisolone Acetate [usp:jan]
49. Pregna-1,4-diene-3,20-dione, 21-(acetyloxy)-11,17-dihydroxy-, (11.beta.)-
50. Econopred (tn)
51. Prednisoloni Acetas
52. Unii-8b2807733d
53. Ncgc00094765-01
54. Prednisolone-acetate
55. Pred Forte (tn)
56. Component Of Metimyd
57. Mfcd00037710
58. Sterane Im And Ia
59. Blephamide S.o.p.
60. Component Of Composone
61. Pred Forte Tm 1%
62. Opera_id_332
63. Pred-g S.o.p.
64. 11beta,17,21-trihydroxypregna-1,4-diene-3,20-dione 21-acetate
65. Schembl7999
66. Chembl1152
67. Component Of Isopto Cetapred
68. 4-08-00-03468 (beilstein Handbook Reference)
69. Mls001148097
70. Mls002207146
71. Mls002548870
72. Component Of Neo-delta-cortef
73. Dtxsid3023502
74. Hms2234f18
75. Hms3259o07
76. Prednisolone Acetate [jan]
77. Prednisolone 21-acetate, >=97%
78. Prednisone Impurity L [ep]
79. 2-((8s,9s,10r,11s,13s,14s,17r)-11,17-dihydroxy-10,13-dimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3h-cyclopenta[a]phenanthren-17-yl)-2-oxoethyl Acetate
80. Bcp18958
81. Hy-b1214
82. Nsc10966
83. Zinc3875348
84. Prednisolone Acetate [vandf]
85. Tox21_111328
86. Prednisolone Acetate [mart.]
87. Pregna-1,4-diene-3,20-dione, 11beta,17,21-trihydroxy-, 21-acetate
88. S2570
89. Prednisolone Acetate [usp-rs]
90. Prednisolone Acetate [who-dd]
91. Prednisolone Acetate [who-ip]
92. Akos007930677
93. Akos015960469
94. Prednisolone 21-acetate [mi]
95. Pregna-1,4-diene-3,20-dione, 11-beta,17,21-trihydroxy-, 21-acetate
96. Pregna-1,4-diene-3,20-dione, 21-(acetyloxy)-11,17-dihydroxy-, (11beta)-
97. Tox21_111328_1
98. Ac-2176
99. Ccg-268673
100. Cs-4642
101. Db15566
102. Nc00470
103. Prednisolone Acetate (jp17/usp/inn)
104. Ncgc00021925-03
105. Ncgc00021925-05
106. Prednisolone Acetate [green Book]
107. 2-((1s,10s,11s,15s,17s,2r,14r)-14,17-dihydroxy-2,15-dimethyl-5-oxotetracyclo[8 .7.0.0<2,7>.0<11,15>]heptadeca-3,6-dien-14-yl)-2-oxoethyl Acetate
108. As-11673
109. Prednisolone Acetate [orange Book]
110. Prednisolone Acetate [ep Monograph]
111. Prednisolone Acetate [usp Impurity]
112. Prednisoloni Acetas [who-ip Latin]
113. Prednisolone Acetate [usp Monograph]
114. Prednisolone Acetate For Peak Identification
115. Metimyd Component Prednisolone Acetate
116. P1283
117. Pred-g Component Prednisolone Acetate
118. Prednisolone Impurity C [ep Impurity]
119. Cetapred Component Prednisolone Acetate
120. Sulphrin Component Prednisolone Acetate
121. Blephamide Component Prednisolone Acetate
122. C08180
123. D00980
124. Poly-pred Component Prednisolone Acetate
125. Predamide Component Prednisolone Acetate
126. Prednisolone Acetate 100 Microg/ml In Methanol
127. Prednisolone Acetate Component Of Pred-g
128. Ab00443788-11
129. Ab00443788_13
130. Prednisolone Acetate 1000 Microg/ml In Methanol
131. Prednisolone Acetate Component Of Cetapred
132. Prednisolone Acetate Component Of Metimyd
133. Prednisolone Acetate Component Of Sulphrin
134. Predsulfair Component Prednisolone Acetate
135. 037p710
136. A828982
137. Prednisolone Acetate Component Of Blephamide
138. Prednisolone Acetate Component Of Poly-pred
139. Prednisolone Acetate Component Of Predamide
140. Prednisolone Acetate Component Of Predsulfair
141. Q-201614
142. Hydrocortisone Acetate Impurity C [ep Impurity]
143. Isopto Cetapred Component Prednisolone Acetate
144. Neo-delta-cortef Component Prednisolone Acetate
145. Q27108063
146. Blephamide S.o.p. Component Prednisolone Acetate
147. Prednisolone Acetate Component Of Isopto Cetapred
148. Prednisolone Acetate Component Of Neo-delta-cortef
149. Methylprednisolone Acetate Impurity E [ep Impurity]
150. Prednisolone 21-acetate, Vetranal(tm), Analytical Standard
151. Prednisolone Acetate Component Of Blephamide S.o.p.
152. 11b,17,21-trihydroxypregna-1,4-diene-3,20-dione 21-acetate
153. Pregna-1,20-dione, 11.beta.,17,21-trihydroxy-, 21-acetate
154. Prednisolone Acetate, British Pharmacopoeia (bp) Reference Standard
155. Prednisolone Acetate, European Pharmacopoeia (ep) Reference Standard
156. (11beta)-11,17-dihydroxy-3,20-dioxopregna-1,4-dien-21-yl Acetate
157. 11.beta.,17,21-trihydroxypregna-1,4-diene-3,20-dione 21-acetate
158. 11.beta.,17-dihydroxy-3,20-dioxopregna-1,4-dien-21-yl Acetate
159. Prednisolone Acetate, United States Pharmacopeia (usp) Reference Standard
160. Pregna-1,20-dione, 21-(acetyloxy)-11,17-dihydroxy-, (11.beta.)-
161. Prednisolone Acetate For Peak Identification, European Pharmacopoeia (ep) Reference Standard
162. Prednisolone Acetate, Pharmaceutical Secondary Standard; Certified Reference Material
163. [2-[(8s,9s,10r,11s,13s,14s,17r)-10,13-dimethyl-11,17-bis(oxidanyl)-3-oxidanylidene-7,8,9,11,12,14,15,16-octahydro-6h-cyclopenta[a]phenanthren-17-yl]-2-oxidanylidene-ethyl] Ethanoate
164. Acetic Acid [2-[(8s,9s,10r,11s,13s,14s,17r)-11,17-dihydroxy-10,13-dimethyl-3-oxo-7,8,9,11,12,14,15,16-octahydro-6h-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl] Ester
| Molecular Weight | 402.5 g/mol |
|---|---|
| Molecular Formula | C23H30O6 |
| XLogP3 | 2.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 4 |
| Exact Mass | 402.20423867 g/mol |
| Monoisotopic Mass | 402.20423867 g/mol |
| Topological Polar Surface Area | 101 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 827 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 7 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 10 | |
|---|---|
| Drug Name | Blephamide s.o.p. |
| PubMed Health | Prednisolone |
| Drug Classes | Endocrine-Metabolic Agent, Immune Suppressant, Ophthalmologic Agent |
| Drug Label | Flo-Pred (prednisolone acetate oral suspension) contains prednisolone which is the acetate ester of the glucocorticoid prednisolone. Glucocorticoids are adrenocortical steroids, both naturally occurring and synthetic, which are readily absorbed from... |
| Active Ingredient | sulfacetamide sodium; Prednisolone acetate |
| Dosage Form | Ointment |
| Route | Ophthalmic |
| Strength | 0.2%; 10% |
| Market Status | Prescription |
| Company | Allergan |
| 2 of 10 | |
|---|---|
| Drug Name | Flo-pred |
| PubMed Health | Prednisolone |
| Drug Classes | Endocrine-Metabolic Agent, Immune Suppressant, Ophthalmologic Agent |
| Drug Label | OMNIPRED (prednisolone acetate ophthalmic suspension) is an adrenocortical steroid product prepared as sterile ophthalmic suspension. The active ingredient is represented by the chemical structure:Established name: Prednisolone AcetateChemical name... |
| Active Ingredient | Prednisolone acetate |
| Dosage Form | Suspension |
| Route | Oral |
| Strength | eq 15mg base/5ml |
| Market Status | Prescription |
| Company | Taro |
| 3 of 10 | |
|---|---|
| Drug Name | Omnipred |
| PubMed Health | Prednisolone (Into the eye) |
| Drug Classes | Ophthalmologic Agent |
| Drug Label | PRED FORTE (prednisolone acetate ophthalmic suspension, USP) 1% is a topical anti-inflammatory agent for ophthalmic use.... |
| Active Ingredient | Prednisolone acetate |
| Dosage Form | Suspension/drops |
| Route | Ophthalmic |
| Strength | 1% |
| Market Status | Prescription |
| Company | Alcon |
| 4 of 10 | |
|---|---|
| Drug Name | Pred forte |
| Drug Label | PRED MILD (prednisolone acetate ophthalmic suspension, USP) 0.12% is a topical anti-inflammatory agent for ophthalmic use. Structural Formula:... |
| Active Ingredient | Prednisolone acetate |
| Dosage Form | Suspension/drops |
| Route | Ophthalmic |
| Strength | 1% |
| Market Status | Prescription |
| Company | Allergan |
| 5 of 10 | |
|---|---|
| Drug Name | Pred mild |
| Active Ingredient | Prednisolone acetate |
| Dosage Form | Suspension/drops |
| Route | Ophthalmic |
| Strength | 0.12% |
| Market Status | Prescription |
| Company | Allergan |
| 6 of 10 | |
|---|---|
| Drug Name | Blephamide s.o.p. |
| PubMed Health | Prednisolone |
| Drug Classes | Endocrine-Metabolic Agent, Immune Suppressant, Ophthalmologic Agent |
| Drug Label | Flo-Pred (prednisolone acetate oral suspension) contains prednisolone which is the acetate ester of the glucocorticoid prednisolone. Glucocorticoids are adrenocortical steroids, both naturally occurring and synthetic, which are readily absorbed from... |
| Active Ingredient | sulfacetamide sodium; Prednisolone acetate |
| Dosage Form | Ointment |
| Route | Ophthalmic |
| Strength | 0.2%; 10% |
| Market Status | Prescription |
| Company | Allergan |
| 7 of 10 | |
|---|---|
| Drug Name | Flo-pred |
| PubMed Health | Prednisolone |
| Drug Classes | Endocrine-Metabolic Agent, Immune Suppressant, Ophthalmologic Agent |
| Drug Label | OMNIPRED (prednisolone acetate ophthalmic suspension) is an adrenocortical steroid product prepared as sterile ophthalmic suspension. The active ingredient is represented by the chemical structure:Established name: Prednisolone AcetateChemical name... |
| Active Ingredient | Prednisolone acetate |
| Dosage Form | Suspension |
| Route | Oral |
| Strength | eq 15mg base/5ml |
| Market Status | Prescription |
| Company | Taro |
| 8 of 10 | |
|---|---|
| Drug Name | Omnipred |
| PubMed Health | Prednisolone (Into the eye) |
| Drug Classes | Ophthalmologic Agent |
| Drug Label | PRED FORTE (prednisolone acetate ophthalmic suspension, USP) 1% is a topical anti-inflammatory agent for ophthalmic use.... |
| Active Ingredient | Prednisolone acetate |
| Dosage Form | Suspension/drops |
| Route | Ophthalmic |
| Strength | 1% |
| Market Status | Prescription |
| Company | Alcon |
| 9 of 10 | |
|---|---|
| Drug Name | Pred forte |
| Drug Label | PRED MILD (prednisolone acetate ophthalmic suspension, USP) 0.12% is a topical anti-inflammatory agent for ophthalmic use. Structural Formula:... |
| Active Ingredient | Prednisolone acetate |
| Dosage Form | Suspension/drops |
| Route | Ophthalmic |
| Strength | 1% |
| Market Status | Prescription |
| Company | Allergan |
| 10 of 10 | |
|---|---|
| Drug Name | Pred mild |
| Active Ingredient | Prednisolone acetate |
| Dosage Form | Suspension/drops |
| Route | Ophthalmic |
| Strength | 0.12% |
| Market Status | Prescription |
| Company | Allergan |
Prednisolone acetate is indicated as an anti-inflammatory or immunosuppressive agent for allergic, dermatologic, gastrointestinal, hematologic, ophthalmologic, nervous system, renal, respiratory, rheumatologic, or infectious conditions. Prednisolone acetate is also indicated in organ transplant patients, as well as endocrine or neoplastic conditions.
Corticosteroids bind to the glucocorticoid receptor, inhibiting pro-inflammatory signals, and promoting anti-inflammatory signals. Prednisolone acetate has a short duration of action as the half life is 2-3 hours. Corticosteroids have a wide therapeutic window as patients make require doses that are multiples of what the body naturally produces. Patients taking corticosteroids should be counselled regarding the risk of hypothalamic-pituitary-adrenal axis suppression and increased susceptibility to infections.
Anti-Inflammatory Agents
Substances that reduce or suppress INFLAMMATION. (See all compounds classified as Anti-Inflammatory Agents.)
Absorption
Prednisolone acetate oral suspension given at a dose equivalent to 15mg prednisolone has a Cmax of 321.1ng/hr, a Tmaxof 1-2 hours, and an AUC of 1999.4ng\*hr/mL. The absorption pharmacokinetics of prednisolone acetate are not significantly different from a comparable dose of prednisolone.
Route of Elimination
Prednisolone acetate is predominantly excreted in the urine.
Volume of Distribution
The volume of distribution of the active metabolite, prednisolone, is 0.22/0.7L/kg.
Clearance
Data regarding the clearance of prednisolone acetate is not readily available.
Prednisolone acetate undergoes ester hydrolysis to [prednisolone]. After this step, the drug undergoes the normal metabolism of prednisolone. Prednisolone can be reversibly metabolized to [prednisone] which is then metabolized to 17,21-dihydroxy-pregnan-1,4,6-trien-3,11,30-trione (M-XVII), 20-dihydro-prednisone (M-V), 6hydroxy-prednisone (M-XII), 6-hydroxy-prednisone (M-XIII), or 20-dihydro-prednisone (M-IV). 20-dihydro-prednisone is metabolized to 17,20,21-trihydroxy-5-pregn-1-en-3,11-dione(M-XVIII). Prednisolone is metabolized to 6-prednisolone (M-XI), 20-dihydro-prednisolone (M-III), 20-dihydro-prednisolone (M-II), 6hydroxy-prednisolone (M-VII), or 6hydroxy-prednisolone(M-VI). 6hydroxy-prednisolone is metabolized to 6,11,17,20,21-pentahydroxypregnan-1,4-diene-3-one (M-X). 6hydroxy-prednisolone is metabolized to 6,11,17,20,21-pentahydroxypregnan-1,4-diene-3-one (M-VIII), 6,11,17,20,21-pentahydroxypregnan-1,4-diene-3-one (M-IX), and 6,11,17,21-tetrahydroxy-5-pregn-1-en-3,20-dione (M-XIV). MVIII is metabolized to 6,11,17,20,21-pentahydroxy-5-pregn-1-en-3-one (M-XV) and then to MXIV, while MIX is metabolized to 6,11,17,20,21-pentahydroxy-5-pregn-1-en-3-one (M-XVI) and then to MXIV. These metabolites and their glucuronide conjugates are excreted predominantly in the urine.
Oral prednisolone acetate has a plasma half life of 2-3 hours.
The short term effects of corticosteroids are decreased vasodilation and permeability of capillaries, as well as decreased leukocyte migration to sites of inflammation. Corticosteroids binding to the glucocorticoid receptor mediates changes in gene expression that lead to multiple downstream effects over hours to days. Glucocorticoids inhibit neutrophil apoptosis and demargination; they inhibit phospholipase A2, which decreases the formation of arachidonic acid derivatives; they inhibit NF-Kappa B and other inflammatory transcription factors; they promote anti-inflammatory genes like interleukin-10. Lower doses of corticosteroids provide an anti-inflammatory effect, while higher doses are immunosuppressive. High doses of glucocorticoids for an extended period bind to the mineralocorticoid receptor, raising sodium levels and decreasing potassium levels.