

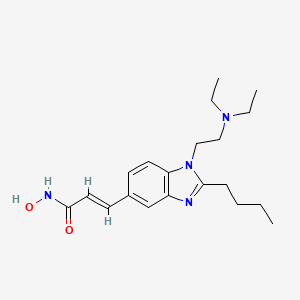

1. 3-(2-butyl-1-(2-diethylaminoethyl)-1h-benzoimidazol-5-yl)-n-hydroxyacrylamide-hydrochloride
2. Sb939 Compound
1. 929016-96-6
2. Sb939
3. Sb 939
4. Pracinostat (sb939)
5. Sb-939
6. Pracinostat [inn]
7. (e)-3-(2-butyl-1-(2-(diethylamino)ethyl)-1h-benzo[d]imidazol-5-yl)-n-hydroxyacrylamide
8. Gpo2jn4uon
9. Chembl1851943
10. 2-propenamide, 3-(2-butyl-1-(2-(diethylamino)ethyl)-1h-benzimidazol-5-yl)-n-hydroxy-, (2e)-
11. Unii-gpo2jn4uon
12. Pracinostatum
13. (2e)-3-[2-butyl-1-[2-(diethylamino)ethyl]-1h-benzimidazol-5-yl]-n-hydroxyacrylamide
14. (2e)-3-{2-butyl-1-[2-(diethylamino)ethyl]-1h-benzimidazol-5-yl}-n-hydroxyacrylamide
15. Sb939,pracinostat
16. Pracinostat(sb939)
17. Sb939 (pracinostat)
18. Pracinostat [who-dd]
19. Mls006011217
20. Schembl833105
21. Gtpl8365
22. Chebi:95071
23. Dtxsid00239196
24. Ex-a351
25. Bcp02073
26. Bdbm50105330
27. Mfcd17215206
28. Nsc758249
29. S1515
30. Zinc43152558
31. Ccg-264903
32. Cs-0567
33. Db05223
34. Nsc-758249
35. (e)-3-[2-butyl-1-[2-(diethylamino)ethyl]benzimidazol-5-yl]-n-hydroxyprop-2-enamide
36. Ncgc00263136-04
37. Ac-33161
38. As-19395
39. Hy-13322
40. Smr004702979
41. Sw219429-1
42. Ec-000.2481
43. A850037
44. Q18344027
45. Kaempferol 3-o-.beta.-d-(6-e-p-coumaroylglucoside)
46. (2e)-3-[2-butyl-1-[2-(diethylamino)ethyl]-1h-benzimidazol-5-yl]-n-hydroxy-2-propenamide
47. (e)-3-[2-butyl-1-(2-diethylaminoethyl)benzimidazol-5-yl]prop-2-enehydroxamic Acid
48. (2e)-3-(2-butyl-1-(2-(dimethylamino)ethyl)-1h-benzimidazol-5-yl)-n-hydroxyprop-2-enamide
49. (2e)-3-{2-butyl-1-[2-(diethylamino)ethyl]-1h-1,3-benzodiazol-5-yl}-n-hydroxyprop-2-enamide
50. (e)-3-(2-butyl-1-(2-diethylaminoethyl)-1h-benzimidazol-5-yl)-n-hydroxy-2-propenamide
| Molecular Weight | 358.5 g/mol |
|---|---|
| Molecular Formula | C20H30N4O2 |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 10 |
| Exact Mass | 358.23687621 g/mol |
| Monoisotopic Mass | 358.23687621 g/mol |
| Topological Polar Surface Area | 70.4 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 453 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of various forms of cancer.
Treatment of acute myeloid leukaemia
SB939 is a novel compound with superior pharmaceutical, metabolic and pharmacokinetic properties. SB939 has demonstrated excellent in vivo anti-tumour activity in various animal models with dose proportional pharmacodynamic effects. The pharmacokinetics and pharmacodynamic attributes of SB939 explain and differentiate it as the best in class HDAC inhibitor.
Absorption
Oral bioavailability in mice is 34%.
Inhibition of HDAC activity allows for the accumulation of acetyl groups on the histone lysine residues resulting in an open chromatin structure and transcriptional activation. In vitro, SB939 causes the accumulation of acetylated histones and induces cell cycle arrest and/or apoptosis of some transformed cells. The mechanism of the antineoplastic effect of SB939 has not been fully characterized.
