


1. 231,514, Ly
2. 231514, Ly
3. Alimta
4. Disodium, Pemetrexed
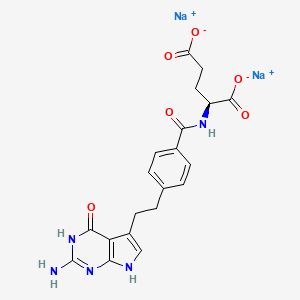
5. Ly 231,514
6. Ly 231514
7. Ly-231,514
8. Ly-231514
9. Ly231514
10. Mta
11. N-(4-(2-(2-amino-3,4-dihydro-4-oxo-7h-pyrrolo(2,3-d)pyrimdin-5-yl)ethyl)benzoyl)glutamic Acid
12. Pemetrexed
1. 150399-23-8
2. Alimta
3. Rolazar
4. Pemetrexed Sodium Salt
5. Tifolar
6. Ly231514 Disodium
7. Chebi:63722
8. Ly231514 Disodium Salt
9. Ly-231514 Disodium Salt
10. Pemetrexed Disodium [usan]
11. 2pku919ba9
12. Pemetrexed Sodium Hydrate
13. Ly-231514
14. N-[4-[2-(2-amino-4,7-dihydro-4-oxo-1h-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]-l-glutamic Acid Disodium Salt
15. Sodium (s)-2-(4-(2-(2-amino-4-oxo-4,7-dihydro-1h-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl)benzamido)pentanedioate
16. Alimta (tn)
17. Pemetrexed (disodium)
18. Pemetrexed Disodium (usan)
19. Disodium;(2s)-2-[[4-[2-(2-amino-4-oxo-3,7-dihydropyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]amino]pentanedioate
20. Ly 231514
21. L-glutamic Acid, N-(4-(2-(2-amino-4,7-dihydro-4-oxo-1h-pyrrolo(2,3-d)pyrimidin-5-yl)ethyl)benzoyl)-, Disodium Salt
22. Pemetrexed-[d5]
23. N-(4-[2-(2-amino-4,7-dihydro-4-oxo-1h-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl)-l-glutamic Acid Disodium Salt
24. Ncgc00167486-01
25. Pemetrexed Disodium, 95%
26. Dsstox_cid_26660
27. Dsstox_rid_81803
28. Unii-2pku919ba9
29. Dsstox_gsid_46660
30. Schembl18348
31. Chembl2360464
32. Dtxsid8046660
33. Ex-a836
34. Hms3264h07
35. Hms3715p06
36. Pemetrexed Sodium Salt [mi]
37. Tox21_112487
38. Bdbm50512141
39. Mfcd07779402
40. Pemetrexed Disodium [usp-rs]
41. Pemetrexed Disodium [who-dd]
42. S1135
43. Akos025149477
44. Akos025392176
45. Ac-1326
46. Ccg-213071
47. Ccg-221272
48. Cs-w004541
49. Ks-5001
50. Pemetrexed Disodium [orange Book]
51. 1129408-57-6
52. Disodium (2s)-2-({4-[2-(2-amino-4-oxo-4,7-dihydro-1h-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl}amino)pentanedioate
53. Pemetrexed Disodium [usp Monograph]
54. Cas-150399-23-8
55. Ly 231,514
56. D03828
57. A809041
58. Pemetrexed Disodium Is Known As A Thymidylate Synthase Inhibitor.
59. Disodium N-(p-(2-((2-amino-4,7-dihydro-4-oxo-1h-pyrrolo(2,3-d)pyrimidin-5-yl)ethyl)benzoyl)-l-glutamate
60. Disodium N-(p-(2-(2-amino-4,7-dihydro-4-oxo-1h-pyrrolo(2,3-d)pyrimidin-5-yl)ethyl)benzoyl)-l-glutamate
61. Disodium N-{4-[2-(2-amino-4-oxo-4,7-dihydro-1h-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl}-l-glutamate
62. N-(4-[2-(2-amino-4,7-dihydro-4-oxo-1h-pyrrolo[2,3-d]pyrimidin-5yl)ethyl]benzoyl)-l-glutamic Acid Disodium Salt
63. N-[4-[2-(2-amino-4,7-dihydro-4-oxo-1h-pyrrolo[2.3-d]pyrimidin-5-yl)ethyl]benzoyl]-l-glutamic Acid Disodium Salt
64. N-[4[2-(-amino-3,4-dihydro-4-oxo-7h-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]-l-glutamate Sodium Salt
65. Sodium (s)-2-(4-(2-(2-amino-4-oxo-4,7-dihydro-3h-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl)benzamido)pentanedioate
66. Sodium(s)-2-(4-(2-(2-amino-4-oxo-4,7-dihydro-1h-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl)benzamido)pentanedioate
| Molecular Weight | 471.4 g/mol |
|---|---|
| Molecular Formula | C20H19N5Na2O6 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 7 |
| Exact Mass | 471.11307191 g/mol |
| Monoisotopic Mass | 471.11307191 g/mol |
| Topological Polar Surface Area | 193 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 737 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
* Malignant pleural mesothelioma:
Pemetrexed Krka in combination with cisplatin is indicated for the treatment of chemotherapy nave patients with unresectable malignant pleural mesothelioma.
* Non-small cell lung cancer :
Pemetrexed Krka in combination with cisplatin is indicated for the first-line treatment of patients with locally advanced or metastatic non-small cell lung cancer other than predominantly squamous cell histology.
Pemetrexed Krka is indicated as monotherapy for the maintenance treatment of locally advanced or metastatic non-small cell lung cancer other than predominantly squamous cell histology in patients whose disease has not progressed immediately following platinum-based chemotherapy.
Pemetrexed Krka is indicated as monotherapy for the second-line treatment of patients with locally advanced or metastatic non-small cell lung cancer other than predominantly squamous cell histology.
* Malignant pleural mesothelioma:
Alimta in combination with cisplatin is indicated for the treatment of chemotherapy-nave patients with unresectable malignant pleural mesothelioma.
* Non-small-cell lung cancer :
Alimta in combination with cisplatin is indicated for the first-line treatment of patients with locally advanced or metastatic non-small-cell lung cancer other than predominantly squamous cell histology.
Alimta is indicated as monotherapy for the maintenance treatment of locally advanced or metastatic non-small-cell lung cancer other than predominantly squamous cell histology in patients whose disease has not progressed immediately following platinum-based chemotherapy.
Alimta is indicated as monotherapy for the second line treatment of patients with locally advanced or metastatic non-small-cell lung cancer other than predominantly squamous cell histology.
Carcinoma of the head and neck (Covered by class waiver: oropharyngeal epithelial carcinoma, excluding nasopharyngeal carcinoma), Malignant pleural mesothelioma
Nucleic Acid Synthesis Inhibitors
Compounds that inhibit cell production of DNA or RNA. (See all compounds classified as Nucleic Acid Synthesis Inhibitors.)
Folic Acid Antagonists
Inhibitors of the enzyme, dihydrofolate reductase (TETRAHYDROFOLATE DEHYDROGENASE), which converts dihydrofolate (FH2) to tetrahydrofolate (FH4). They are frequently used in cancer chemotherapy. (From AMA, Drug Evaluations Annual, 1994, p2033) (See all compounds classified as Folic Acid Antagonists.)
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
L01BA04
L01BA04

