
1. Pat-1251
2. 2007885-39-2
3. Y3hmf6g24b
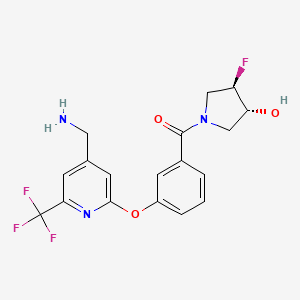
4. [3-[4-(aminomethyl)-6-(trifluoromethyl)pyridin-2-yl]oxyphenyl]-[(3r,4r)-3-fluoro-4-hydroxypyrrolidin-1-yl]methanone
5. (3-((4-(aminomethyl)-6-(trifluoromethyl)-2-pyridinyl)oxy)phenyl)((3r,4r)-3-fluoro-4-hydroxy-1-pyrrolidinyl)methanone
6. 2098884-52-5
7. Methanone, (3-((4-(aminomethyl)-6-(trifluoromethyl)-2-pyridinyl)oxy)phenyl)((3r,4r)-3-fluoro-4-hydroxy-1-pyrrolidinyl)-
8. Chembl4116649
9. Schembl18072268
10. Us10774069, Compound 1
11. Bdbm461421
12. Ex-a2634
13. Hy-107422
14. Cs-0028450
15. J3.653.610a
16. [3-[[4-(aminomethyl)-6-(trifluoromethyl)-2-pyridyl]oxy]phenyl][(3r,4r)-3-fluoro-4-hydroxy-1-pyrrolidinyl]methanone
17. Rel-(3-((4-(aminomethyl)-6-(trifluoromethyl)pyridin-2-yl)oxy)phenyl)((3r,4r)-3-fluoro-4-hydroxypyrrolidin-1-yl)methanone
| Molecular Weight | 399.3 g/mol |
|---|---|
| Molecular Formula | C18H17F4N3O3 |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 4 |
| Exact Mass | 399.12060406 g/mol |
| Monoisotopic Mass | 399.12060406 g/mol |
| Topological Polar Surface Area | 88.7 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 551 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
