
1. 1491-59-4
2. Oxymethazoline
3. Hazol
4. Oximetazolinum
5. Oxylazine
6. Rhinofrenol
7. Sinerol
8. Iliadin
9. Navisin
10. Nezeril
11. Nafrine
12. Oxymetozoline
13. Oximetazolina
14. Oxymetazolinum
15. Afrin
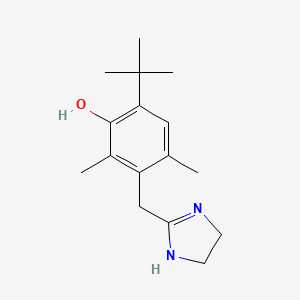
16. Phenol, 3-[(4,5-dihydro-1h-imidazol-2-yl)methyl]-6-(1,1-dimethylethyl)-2,4-dimethyl-
17. 6-tert-butyl-3-(4,5-dihydro-1h-imidazol-2-ylmethyl)-2,4-dimethylphenol
18. Nasacon
19. Operil
20. 6-t-butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimethylphenol
21. 2-(4-tert-butyl-2,6-dimethyl-3-hydroxybenzyl)-2-imidazoline
22. 6-tert-butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimethylphenol
23. H 990
24. 8vln5b44zy
25. 3-[(4,5-dihydro-1h-imidazol-2-yl)methyl]-6-(1,1-dimethylethyl)-2,4-dimethylphenol
26. Dtxsid3040691
27. Phenol, 6-tert-butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimethyl-
28. Phenol, 3-((4,5-dihydro-1h-imidazol-2-yl)methyl)-6-(1,1-dimethylethyl)-2,4-dimethyl-
29. Chebi:7862
30. Dtxcid1020691
31. Phenol, 6-t-butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimethyl-
32. 3-((4,5-dihydro-1h-imidazol-2-yl)methyl)-6-(1,1-dimethylethyl)-2,4-dimethylphenol
33. Refchem:6090
34. A14aa05
35. R01aa05
36. R01ab07
37. S01ga04
38. Sinufrin Quick Relief Decongestant
39. 216-079-1
40. Rhinolitan
41. Hsdb 3143
42. Oxymetazoline (inn)
43. Navasin
44. Ncgc00015766-08
45. 6-tert-butyl-3-[(4,5-dihydro-1h-imidazol-2-yl)methyl]-2,4-dimethylphenol
46. Oxymetazoline [inn]
47. Oxymetazoline [inn:ban]
48. Oxymetazolinum [inn-latin]
49. Oximetazolina [inn-spanish]
50. Cas-1491-59-4
51. Operil (tn)
52. Einecs 216-079-1
53. Oxymetazoline Hydrochloride Crystalline
54. Unii-8vln5b44zy
55. Brn 0886303
56. Oxymetazolina
57. 6-(tert-butyl)-3-((4,5-dihydro-1h-imidazol-2-yl)methyl)-2,4-dimethylphenol
58. 6-(tert-butyl)-3-[(4,5-dihydro-1h-imidazol-2-yl)methyl]-2,4-dimethylphenol
59. Mfcd00242798
60. Nasivine (salt/mix)
61. Spectrum_001051
62. Tocris-1142
63. 6-tert-butyl-3-(4,5-dihydro-1h-imidazol-2-ylmethyl)-2,4-dimethyl-phenol;hydrochloride
64. Prestwick0_000224
65. Prestwick1_000224
66. Prestwick2_000224
67. Prestwick3_000224
68. Spectrum2_000998
69. Spectrum3_000533
70. Spectrum4_000464
71. Spectrum5_001114
72. Lopac-o-2378
73. Oxymetazoline [mi]
74. Biomol-nt_000161
75. Chembl762
76. Oxymetazoline [hsdb]
77. Lopac0_000903
78. Schembl24301
79. Bspbio_000267
80. Bspbio_002145
81. Gtpl124
82. Kbiogr_000908
83. Kbioss_001531
84. Oxymetazoline [vandf]
85. Cid_66259
86. Divk1c_000567
87. Spbio_001095
88. Spbio_002188
89. Oxymetazoline [who-dd]
90. Bpbio1_000295
91. Bpbio1_000419
92. Orb1685732
93. Schembl29825106
94. Bdbm30712
95. Kbio1_000567
96. Kbio2_001531
97. Kbio2_004099
98. Kbio2_006667
99. Kbio3_001645
100. Ninds_000567
101. Hms2089g03
102. Albb-036521
103. Tox21_110217
104. Ebc-26354
105. Stk075254
106. 3-(4,5-dihydro-1h-imidazol-2-ylmethyl)-2,4-dimethyl-6-tert-butyl-phenol
107. Akos007930348
108. Tox21_110217_1
109. Ac-6370
110. Cas-151615
111. Ccg-204985
112. Db00935
113. Sdccgsbi-0050878.p005
114. Idi1_000567
115. Ncgc00015766-01
116. Ncgc00015766-02
117. Ncgc00015766-03
118. Ncgc00015766-04
119. Ncgc00015766-05
120. Ncgc00015766-06
121. Ncgc00015766-07
122. Ncgc00015766-09
123. Ncgc00015766-10
124. Ncgc00015766-11
125. Ncgc00015766-13
126. Ncgc00015766-16
127. Ncgc00015766-21
128. Ncgc00022345-02
129. Ncgc00022345-04
130. Ncgc00022345-05
131. Ncgc00022345-06
132. Hy-12722
133. Sy278685
134. Sbi-0050878.p004
135. Cs-0012298
136. H-990
137. Ns00005342
138. C07363
139. D08322
140. G78135
141. Ab00053513-12
142. Ab00053513-13
143. Ab00053513_14
144. Ab00053513_15
145. En300-23521946
146. L000459
147. Q417813
148. Brd-k16195444-001-01-7
149. Brd-k16195444-003-16-1
150. Brd-k16195444-003-24-5
151. Brd-k16195444-003-25-2
152. Brd-k16195444-003-26-0
153. 6-tert-butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimethyl-phenol;hydrochloride
154. 6-tert-butyl-3-(4,5-dihydro-1h-imidazol-2-ylmethyl)-2,4-dimethylphenol #
155. Phenol, 6-tert-butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimethyl- (7ci,8ci)
156. Phenol, 3-[(4,5-dihydro-1h-imidazol-2-yl)methyl]-6-(1,1-dimethylethyl)-2,4-dimethyl- (9ci)
| Molecular Weight | 260.37 g/mol |
|---|---|
| Molecular Formula | C16H24N2O |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 3 |
| Exact Mass | Da |
| Monoisotopic Mass | Da |
| Topological Polar Surface Area | 44.6 |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 345 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Adrenergic alpha-Agonists; Nasal Decongestants; Sympathomimetics
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Adrenergic (vasoconstrictor); nasal decongestant
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 1247
Oxymetazoline /ophthalmic/ is indicated for temporary relief of redness associated with minor irritations of the eye, such as those caused by pollen-related allergies colds, dust, smog, wind, swimming, or wearing contact lenses. /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2320
Nasal oxymetazoline is used for the relief of sinus congestion. /NOT included in US product labeling/
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 2261
For more Therapeutic Uses (Complete) data for OXYMETAZOLINE (11 total), please visit the HSDB record page.
Intranasal use of oxymetazoline may occasionally cause systemic sympathomimetic effects such as hypertension, nervousness, nausea, dizziness, headache, insomnia, palpitation, or reflex bradycardia.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 2824
Intranasal administration of oxymetazoline may cause transient burning, stinging, increased nasal discharge or dryness of the nasal mucosa, and sneezing. Rebound congestion, characterized by chronic redness, swelling, and rhinitis, frequently occurs with prolonged use and may result in overuse of the drug. Prolonged use of nasal decongestant solutions should be avoided for these reasons.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 2824
The incidence of serious adverse effects is low in patients receiving therapeutic dosages of topical oxymetazoline hydrochloride.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 2824
Safe use of oxymetazoline during pregnancy has not been established. Oxymetazoline hydrochloride ophthalmic or nasal solutions should be used during pregnancy only when instructed by a clinician.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 2824
For more Drug Warnings (Complete) data for OXYMETAZOLINE (13 total), please visit the HSDB record page.
Oxymetazoline is indicated for the topical treatment of persistent facial erythema associated with rosacea in adults. Ophthalmic oxymetazoline is indicated for the treatment of acquired blepharoptosis in adults. When used in combination with tetracaine intranasally, oxymetazoline is indicated for regional anesthesia when performing a restorative procedure on Teeth 4-13 and A-J in adults and children who weigh 40 kg or more. Oxymetazoline can be found in over-the-counter nasal products as a nasal decongestant. For off-label uses, oxymetazoline has been used during nasal intubation and during ear, nose, and throat surgery to improve visualization of the airway and to minimize post-operative bleeding.
Adrenergic alpha-Agonists
Drugs that selectively bind to and activate alpha adrenergic receptors.
Sympathomimetics
Drugs that mimic the effects of stimulating postganglionic adrenergic sympathetic nerves. Included here are drugs that directly stimulate adrenergic receptors and drugs that act indirectly by provoking the release of adrenergic transmitters.
Nasal Decongestants
Drugs designed to treat inflammation of the nasal passages, generally the result of an infection (more often than not the common cold) or an allergy related condition, e.g., hay fever. The inflammation involves swelling of the mucous membrane that lines the nasal passages and results in inordinate mucus production. The primary class of nasal decongestants are vasoconstrictor agents. (From PharmAssist, The Family Guide to Health and Medicine, 1993)
R01AA05
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (2021) DOI:10.1021/acsenvironau.1c00008. List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
S - Sensory organs
S01 - Ophthalmologicals
S01G - Decongestants and antiallergics
S01GA - Sympathomimetics used as decongestants
S01GA04 - Oxymetazoline
R - Respiratory system
R01 - Nasal preparations
R01A - Decongestants and other nasal preparations for topical use
R01AA - Sympathomimetics, plain
R01AA05 - Oxymetazoline
R - Respiratory system
R01 - Nasal preparations
R01A - Decongestants and other nasal preparations for topical use
R01AB - Sympathomimetics, combinations excl. corticosteroids
R01AB07 - Oxymetazoline
D - Dermatologicals
D11 - Other dermatological preparations
D11A - Other dermatological preparations
D11AX - Other dermatologicals
D11AX27 - Oxymetazoline
ATCvet Code
QD - Dermatologicals
QD11 - Other dermatological preparations
QD11A - Other dermatological preparations
QD11AX - Other dermatologicals
QD11AX27 - Oxymetazoline
ATCvet Code
QR - Respiratory system
QR01 - Nasal preparations
QR01A - Decongestants and other nasal preparations for topical use
QR01AA - Sympathomimetics, plain
QR01AA05 - Oxymetazoline
ATCvet Code
QR - Respiratory system
QR01 - Nasal preparations
QR01A - Decongestants and other nasal preparations for topical use
QR01AB - Sympathomimetics, combinations excl. corticosteroids
QR01AB07 - Oxymetazoline
ATCvet Code
QS - Sensory organs
QS01 - Ophthalmologicals
QS01G - Decongestants and antiallergics
QS01GA - Sympathomimetics used as decongestants
QS01GA04 - Oxymetazoline
Absorption
Imidazole derivatives such as oxymetazoline are readily absorbed across mucosal membranes, especially in children. In adult subjects with erythema associated with rosacea, the mean standard deviation (SD) Cmax was 60.5 53.9 pg/mL and the AUC from time 0 to 24 hours (AUC0-24hr) was 895 798 pg x hr/mL following topical administration of first-dose oxymetazoline. Following once-daily topical applications for 28 days, the mean SD Cmax was 66.4 67.1 pg/mL and the AUC0-24hr was 1050 992 pg x hr/mL. Following twice-daily applications for 28 days, the mean SD Cmax was 68.8 61.1 pg/mL and the AUC0-24hr was 1530 922 pg x hr/mL. Following single-drop ocular administration of oxymetazoline in healthy adult subjects, the mean SD Cmax was 30.5 12.7 pg/mL and the area under the concentration-time curve (AUCinf) was 468 214 pg x hr/mL. The median Tmax was 2 hours, ranging from 0.5 to 12 hours. Following nasal administration of an 0.6 mL combination product containing tetracaine and oxymetazoline in adult subjects, the maximum concentrations of oxymetazoline were reached within approximately 10 minutes. The mean Cmax was 1.78 ng/mL and the AUC0-inf value was 4.24 ng x h/mL, with a median Tmax of 5 minutes.
Route of Elimination
While the excretion of oxymetazoline following nasal, topical, or ophthalmic administration of oxymetazoline has not been fully characterized in humans, it is believed that the predominant route of elimination at clinically relevant concentrations of oxymetazoline is renal excretion.
Volume of Distribution
There is limited information on the volume of distribution of oxymetazoline.
Clearance
There is limited information on the clearance rate of oxymetazoline.
... Oxymetazoline /at an optimum strength of 0.025%/ was absorbed slowly into the eye: only 0.006% of the original drug concentration was found in the aqueous humors of rabbits 30 minutes after instillation; the balance remained primarily in external ocular tissues. Metabolic studies in rabbits indicated that excreted amounts of unmetabolized radioactive oxymetazoline in urine following drug administration were similar (23%) for the ocular and nasal routes of application. The proportions of oxymetazoline metabolite to unchanged oxymetazoline were constant for all administration routes tested.
PMID:6347152 Duzman F et al; Arch Ophthalmol 101 (7): 1122-6 (1983).
Following intranasal application of oxymetazoline hydrochloride solutions, local vasoconstriction usually occurs within 5-10 minutes and persists for 5-6 hours with a gradual decline over the next 6 hours. Following topical application of oxymetazoline hydrochloride ophthalmic solution, local vasoconstriction usually occurs within minutes and may persist for up to 6 hours. Occasionally, enough oxymetazoline may be absorbed to produce systemic effects.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 2825
_In vitro_, oxymetazoline was minimally metabolized by human liver enzymes to produce mono-oxygenated and dehydrogenated metabolites. About 95.9% of the total dose of oxymetazoline remained as an unchanged parent compound after a 120-minute incubation with human liver microsomes. When incubated in rat, rabbit, and human liver post-mitochondrial supernatant fraction from homogenized tissue (S9) fractions, oxymetazoline was more efficiently metabolized by rabbit liver S9 fractions (~65%) than by rat (~20%) or human (~10%) liver S9 fractions. At concentrations (50 M) at least 130-fold greater than the usual therapeutic intranasal dose (400 nM), CYP2C19 was suggested to be involved in the oxidation of oxymetazoline following intranasal administration; however, metabolites in humans have not been fully characterized up to date and remain speculated based on _in vitro_ studies using rat and rabbit liver S9 fractions and microsomes. The O-glucuronide metabolite catalyzed by UGT1A9 has been identified _in vitro_.
Following ocular administration in healthy adults, the mean terminal half-life was 8.3 hours, ranging from 5.6 to 13.9 hours. The terminal half-life of oxymetazoline following nasal administration of the combination product containing tetracaine and oxymetazoline in adult subjects is approximately 5.2 hours.
Oxymetazoline binds to 1- and 2-adrenoceptors, which are Gq- and Gi-protein-coupled receptors respectively. 1-adrenoceptors agonism promotes vascular smooth muscle contraction by increasing intracellular calcium levels through activating phospholipase C, while 2-adrenoceptors agonism, specifically the 2B-adrenoceptors, can also elicit vasoconstriction through the inhibition of adenyl cyclase. Rosacea is a condition characterized by transient and persistent facial erythema. By stimulating 1A-adrenoceptors and causing vasoconstriction, oxymetazoline is believed to diminish the symptoms of erythema. In blepharoptosis, it is hypothesized that oxymetazoline works by stimulating -adrenergic receptors on the Mller muscle that elevates the upper eyelid, causing muscle contraction. Oxymetazoline is used in combination with tetracaine for local anesthesia in dentistry. Such combination use adds beneficial effects: the vasoconstrictor counteracts the local anesthetic agent's vasodilatory action, thereby constricting dilated arterioles and reducing blood flow to the application area. Oxymetazoline relieves nasal congestion by vasoconstricting the respiratory microvasculature, in both resistance and capacitance blood vessels on the human nasal mucosa, leading to decreased nasal mucosal blood flow, edema, and airflow resistance.
The mechanism of action of oxymetazoline has not been conclusively determined. Most pharmacologists believe that the drug directly stimulates a-adrenergic receptors of the sympathetic nervous system and exerts little or no effect on beta-adrenergic receptors. Intranasal application of oxymetazoline results in constriction of dilated arterioles and reduction in nasal blood flow and congestion. In addition, obstructed eustachian ostia may be opened. Nasal ventilation and aeration are improved temporarily; however, rebound vasodilation and congestion usually occur to some degree. Following topical application of oxymetazoline hydrochloride ophthalmic solution, conjunctival congestion is temporarily relieved, but overuse of the drug may produce rebound hyperemia.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 2824