

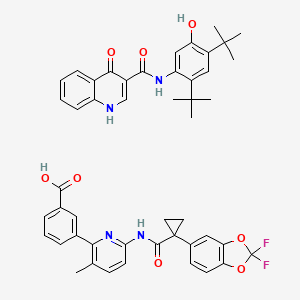

1. Ivacaftor - Lumacaftor
2. Ivacaftor, Lumacaftor Drug Combination
3. Lumacaftor, Ivacaftor Drug Combination
1. Lumacaftor / Ivacaftor
2. Lumacaftor And Ivacaftor
3. Lumacaftor And Ivacaftor Tablet
4. Schembl19410545
5. Vx 700-vx 809 Combination
6. Vx 809-vx 770 Combination
7. Ivacaftor Mixture With Lumacaftor
8. Lumacaftor, Ivacaftor Drug Combination
9. S900006790
10. 1815566-23-4
| Molecular Weight | 844.9 g/mol |
|---|---|
| Molecular Formula | C48H46F2N4O8 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 9 |
| Exact Mass | 844.32837076 g/mol |
| Monoisotopic Mass | 844.32837076 g/mol |
| Topological Polar Surface Area | 176 Ų |
| Heavy Atom Count | 62 |
| Formal Charge | 0 |
| Complexity | 1450 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 1 | |
|---|---|
| Drug Name | ORKAMBI |
| Active Ingredient | IVACAFTOR; LUMACAFTOR |
| Company | VERTEX PHARMS INC (Application Number: N206038. Patents: 7495103, 7973038, 8324242, 8410274, 8507534, 8653103, 8716338, 8741933, 8754224, 8846718, 8993600, 9150552, 9192606, 9216969, 9670163, 9931334) |
Orkambi tablets are indicated for the treatment of cystic fibrosis (CF) in patients aged 6 years and older who are homozygous for the F508del mutation in the CFTR gene.
Orkambi granules are indicated for the treatment of cystic fibrosis (CF) in children aged 2 years and older who are homozygous for the F508del mutation in the CFTR gene.
R07AX30
