


1. L-1037536
2. Mk-0822
1. 603139-19-1
2. Mk-0822
3. Odanacatib (mk-0822)
4. Mk0822
5. Mk 0822
6. Odanacatib (mk0822)
7. Odanacatib (mk 0822)
8. Chembl481611
9. N673f6w2vh
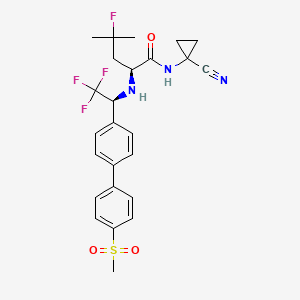
10. (s)-n-(1-cyanocyclopropyl)-4-fluoro-4-methyl-2-(((s)-2,2,2-trifluoro-1-(4'-(methylsulfonyl)-[1,1'-biphenyl]-4-yl)ethyl)amino)pentanamide
11. (2s)-n-(1-cyanocyclopropyl)-4-fluoro-4-methyl-2-[[(1s)-2,2,2-trifluoro-1-[4-(4-methylsulfonylphenyl)phenyl]ethyl]amino]pentanamide
12. (2s)-n-(1-cyanocyclopropyl)-4-fluoro-4-methyl-2-[[(1s)-2,2,2-trifluoro-1-[4'-(methylsulfonyl)[1,1'-biphenyl]-4-yl]ethyl]amino]pentanamide
13. (s)-n-(1-cyanocyclopropyl)-4-fluoro-4-methyl-2-((s)-2,2,2-trifluoro-1-(4'-(methylsulfonyl)biphenyl-4-yl)ethylamino)pentanamide
14. Odanacatib [usan]
15. Odanacatib [usan:inn]
16. Unii-n673f6w2vh
17. (2s)-n-(1-cyanocyclopropyl)-4-fluoro-4-methyl-2-(((1s)-2,2,2-trifluoro-1-(4'-(methylsulfonyl)(1,1'-biphenyl)-4-yl)ethyl)amino)pentanamide
18. Mk-0822a
19. Odanacatib [mi]
20. Odanacatib [inn]
21. Odanacatib [jan]
22. Odanacatib (jan/usan)
23. Odanacatib [mart.]
24. Odanacatib [who-dd]
25. Mls006010197
26. Gtpl6478
27. L-1037536
28. Schembl1496266
29. Dtxsid40209075
30. Ex-a552
31. Bcpp000141
32. Cas:603139-19-1;odanacatib
33. Bdbm50255753
34. Mfcd11042419
35. Nsc766811
36. S1115
37. Zinc42893657
38. Akos015900719
39. Bcp9001020
40. Ccg-269888
41. Cs-0277
42. Db06670
43. Nsc-766811
44. Ncgc00346637-01
45. Ac-27468
46. As-19562
47. Hy-10042
48. Smr004676504
49. Sw219669-1
50. D08955
51. Mk-0822;mk 0822;mk0822
52. 139m191
53. J-690332
54. Q2014070
55. N-(1-cyanocyclopropyl)-4-fluoro-n2-{(1s)-2,2,2-trifluoro-1-[4'-(methylsulfonyl)biphenyl-4-yl]ethyl}-l-leucinamide
56. N1-(1-cyanocyclopropyl)-4-fluoro-n2-{(1s)-2,2,2-trifluoro-1-[4'-(methyl Sulfonyl)-1,1'-biphenyl-4-yl]ethyl}-l-leucinamide
57. N1-(1-cyanocyclopropyl)-4-fluoro-n2-{(1s)-2,2,2-trifluoro-1-[4'-(methylsulfonyl)-1,1'-biphenyl-4-yl]ethyl}-l-leucinamide
58. Pentanamide, N-(1-cyanocyclopropyl)-4-fluoro-4-methyl-2-(((1s)-2,2,2-trifluoro-1-(4'-(methylsulfonyl)(1,1'-biphenyl)-4-yl)ethyl)amino)-, (2s)-
| Molecular Weight | 525.6 g/mol |
|---|---|
| Molecular Formula | C25H27F4N3O3S |
| XLogP3 | 4.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 9 |
| Exact Mass | 525.17092555 g/mol |
| Monoisotopic Mass | 525.17092555 g/mol |
| Topological Polar Surface Area | 107 Ų |
| Heavy Atom Count | 36 |
| Formal Charge | 0 |
| Complexity | 934 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for use/treatment in osteoporosis.
Treatment of osteoporosis
Increases bone mineral density and reduces risk of fractures in osteoporosis.
Absorption
Tmax of 2-6h. The absolute bioavailabilities observed with 30mg and 50 mg doses are 70% and 30% respectively. When taken with high fat meals the 50mg dose's bioavailability increases to 49% and tmax increases to 10.5h.
Route of Elimination
16.9% excreted in urine. 74.5% excreted in feces.
Volume of Distribution
100L
Clearance
Total clearance of 0.8L/h.
The major metabolite is the product of hydroxylation by CYP3A4 and CYP2C8. This metabolite is active but is 25 times less effective at inhibiting cathepsin K than odanacatib. The other metabolites are produced through glutathione conjugation, hydrolysis, dealkylation, glucuronidation, oxidation, and cyclization.
Apparent half life observed to be 87.3-94.7h.
Odanacatib inhibits cathepsin K, likely by binding to its active site. Cathepsin K is a cysteine protease enzyme which is secreted by osteoclasts. Cathepsin K is responsible for the breakdown of collagen in the bone matrix as part of bone resorption. The inhibition of this enzyme results in decreased bone resorption without affecting bone deposition resulting in increased bone mineral density. This increased bone mineral density strengthens the bone which leads to fewer fractures in osteoporosis.
