


1. Esomeprazole
2. Esomeprazole Potassium
3. Esomeprazole Sodium
4. Esomeprazole Strontium
5. Esomeprazole Strontium Anhydrous
6. Nexium
7. Strontium, Esomeprazole
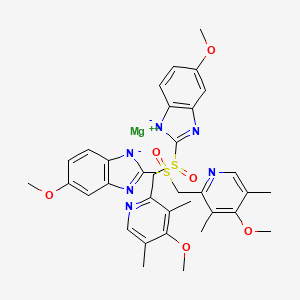
1. Omeprazole Magnesium
2. 161973-10-0
3. Prilosec Otc
4. H 168/68 Magnesium
5. 95382-33-5
6. Omeprazole (as Magnesium)
7. 426qfe7xlk
8. Magnesium;5-methoxy-2-[(4-methoxy-3,5-dimethylpyridin-2-yl)methylsulfinyl]benzimidazol-1-ide
9. Esomeprazole Magnesium Salt
10. Unii-426qfe7xlk
11. Omeprazole Magnesium [usan]
12. Omeprazole Magnesium [usan:usp]
13. Prilosec Otc (tn)
14. Esomeprazole(magnesium)
15. Omeprazole Magnesium Salt
16. Omeprazole Magnesium (usp)
17. Mls001165732
18. Schembl722792
19. Esomeprazole Magnesium (nexium)
20. Chembl1567328
21. Chebi:94401
22. H-168/68 Magnesium
23. Hms2878h13
24. Omeprazole Magnesium [vandf]
25. Mfcd06798050
26. Omeprazole Magnesium [mart.]
27. Omeprazole Magnesium [usp-rs]
28. Omeprazole Magnesium [who-dd]
29. Akos015896379
30. Akos025402081
31. Omeprazole Magnesium Salt [mi]
32. As-75082
33. Omeprazole Magnesium [orange Book]
34. Smr000550477
35. Omeprazole Magnesium [ep Monograph]
36. Omeprazole Magnesium [usp Impurity]
37. Omeprazole Magnesium [usp Monograph]
38. Ft-0657297
39. Sw220306-1
40. Talicia Component Omeprazole Magnesium
41. D05259
42. Omeprazole Magnesium Component Of Talicia
43. A810316
44. J-014249
45. Q-100195
46. Q27166253
47. 5-methoxy-1h-1,3-benzimidazol-2-yl (4-methoxy-3,5-dimethyl-2-pyridinyl)methyl Sulfoxide
48. (rs)-5-methoxy-2-(((4-methoxy-3,5-dimethyl-2-pyridinyl)methyl)sulfinyl)-1h-benzimidazole, Magnesium Salt (2:1)
49. 5-methoxy-2-(((4-methoxy-3,5-dimethyl-2-pyridinyl)methyl)sulfinyl)-1h-benzimidazole, Magnesium Salt
50. 5-methoxy-2-(((4-methoxy-3,5-dimethyl-2-pyridyl)methyl)sulfinyl)benzimidazole, Magnesium Salt (2:1)
51. Magnesium 5-methoxy-2-[(4-methoxy-3,5-dimethyl-2-pyridyl)methylsulfinyl]benzimidazol-1-ide;esomeprazole Magnesium(random Configuration)
52. Magnesium(2+) 5-methoxy-2-[(4-methoxy-3,5-dimethylpyridin-2-yl)methanesulfinyl]-1h-1,3-benzodiazol-1-ide 6-methoxy-2-[(4-methoxy-3,5-dimethylpyridin-2-yl)methanesulfinyl]-1h-1,3-benzodiazol-1-ide
53. Magnesium, Bis(6-methoxy-2-(((4-methoxy-3,5-dimethyl-2-pyridinyl)methyl)sulfinyl-.kappa.o)-1h-benzimidazolato-.kappa.n3)-, (t-4)-
| Molecular Weight | 713.1 g/mol |
|---|---|
| Molecular Formula | C34H36MgN6O6S2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 14 |
| Rotatable Bond Count | 10 |
| Exact Mass | 712.1988169 g/mol |
| Monoisotopic Mass | 712.1988169 g/mol |
| Topological Polar Surface Area | 163 Ų |
| Heavy Atom Count | 49 |
| Formal Charge | 0 |
| Complexity | 453 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
| 1 of 6 | |
|---|---|
| Drug Name | Esomeprazole magnesium |
| PubMed Health | Esomeprazole |
| Drug Classes | Gastric Acid Secretion Inhibitor, Gastrointestinal Agent |
| Drug Label | The active ingredient in NEXIUM (esomeprazole magnesium) Delayed-Release Capsules and NEXIUM (esomeprazole magnesium) For Delayed-Release Oral Suspension is bis(5-methoxy-2-[(S)-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole... |
| Active Ingredient | Esomeprazole magnesium |
| Dosage Form | Capsule, delayed release |
| Route | oral |
| Strength | 40mg; 20mg |
| Market Status | Tentative Approval |
| Company | Ranbaxy |
| 2 of 6 | |
|---|---|
| Drug Name | Nexium |
| Drug Label | The active ingredient in NEXIUM (esomeprazole magnesium) Delayed-Release Capsules and NEXIUM (esomeprazole magnesium) For Delayed-Release Oral Suspension is bis(5-methoxy-2-[(S)-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole... |
| Active Ingredient | Esomeprazole magnesium |
| Dosage Form | Capsule, delayed rel pellets; For suspension, delayed release |
| Route | Oral |
| Strength | eq 5mg base/packet; eq 20mg base/packet; eq 20mg base; eq 40mg base; eq 10mg base/packet; eq 40mg base/packet; eq 2.5mg base/packet |
| Market Status | Prescription |
| Company | Astrazeneca |
| 3 of 6 | |
|---|---|
| Drug Name | Omeprazole magnesium |
| Drug Label | The active ingredient in NEXIUM (esomeprazole magnesium) Delayed-Release Capsules and NEXIUM (esomeprazole magnesium) For Delayed-Release Oral Suspension is bis(5-methoxy-2-[(S)-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole... |
| Active Ingredient | Omeprazole magnesium |
| Dosage Form | Capsule, delayed release |
| Route | Oral |
| Strength | eq 20mg base |
| Market Status | Over the Counter |
| Company | Dr Reddys Labs |
| 4 of 6 | |
|---|---|
| Drug Name | Esomeprazole magnesium |
| PubMed Health | Esomeprazole |
| Drug Classes | Gastric Acid Secretion Inhibitor, Gastrointestinal Agent |
| Drug Label | The active ingredient in NEXIUM (esomeprazole magnesium) Delayed-Release Capsules and NEXIUM (esomeprazole magnesium) For Delayed-Release Oral Suspension is bis(5-methoxy-2-[(S)-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole... |
| Active Ingredient | Esomeprazole magnesium |
| Dosage Form | Capsule, delayed release |
| Route | oral |
| Strength | 40mg; 20mg |
| Market Status | Tentative Approval |
| Company | Ranbaxy |
| 5 of 6 | |
|---|---|
| Drug Name | Nexium |
| Drug Label | The active ingredient in NEXIUM (esomeprazole magnesium) Delayed-Release Capsules and NEXIUM (esomeprazole magnesium) For Delayed-Release Oral Suspension is bis(5-methoxy-2-[(S)-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole... |
| Active Ingredient | Esomeprazole magnesium |
| Dosage Form | Capsule, delayed rel pellets; For suspension, delayed release |
| Route | Oral |
| Strength | eq 5mg base/packet; eq 20mg base/packet; eq 20mg base; eq 40mg base; eq 10mg base/packet; eq 40mg base/packet; eq 2.5mg base/packet |
| Market Status | Prescription |
| Company | Astrazeneca |
| 6 of 6 | |
|---|---|
| Drug Name | Omeprazole magnesium |
| Drug Label | The active ingredient in NEXIUM (esomeprazole magnesium) Delayed-Release Capsules and NEXIUM (esomeprazole magnesium) For Delayed-Release Oral Suspension is bis(5-methoxy-2-[(S)-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole... |
| Active Ingredient | Omeprazole magnesium |
| Dosage Form | Capsule, delayed release |
| Route | Oral |
| Strength | eq 20mg base |
| Market Status | Over the Counter |
| Company | Dr Reddys Labs |
Anti-Ulcer Agents
Various agents with different action mechanisms used to treat or ameliorate PEPTIC ULCER or irritation of the gastrointestinal tract. This has included ANTIBIOTICS to treat HELICOBACTER INFECTIONS; HISTAMINE H2 ANTAGONISTS to reduce GASTRIC ACID secretion; and ANTACIDS for symptomatic relief. (See all compounds classified as Anti-Ulcer Agents.)
Proton Pump Inhibitors
Compounds that inhibit H(+)-K(+)-EXCHANGING ATPASE. They are used as ANTI-ULCER AGENTS and sometimes in place of HISTAMINE H2 ANTAGONISTS for GASTROESOPHAGEAL REFLUX. (See all compounds classified as Proton Pump Inhibitors.)

