


1. 839713-36-9
2. Apd125
3. Apd-125
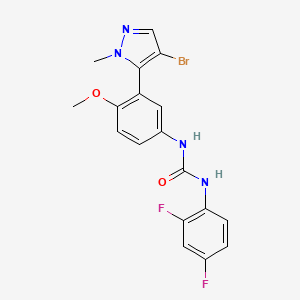
4. 1-[3-(4-bromo-2-methylpyrazol-3-yl)-4-methoxyphenyl]-3-(2,4-difluorophenyl)urea
5. 1-[3-(4-bromo-1-methyl-1h-pyrazol-5-yl)-4-methoxyphenyl]-3-(2,4-difluorophenyl)urea
6. 1-[3-(4-bromo-2-methyl-2h-pyrazol-3-yl)-4-methoxyphenyl]-3-(2,4-difluorophenyl)urea
7. 4za73qew2p
8. Chembl598172
9. 1-(3-(4-bromo-1-methyl-1h-pyrazol-5-yl)-4-methoxyphenyl)-3-(2,4-difluorophenyl)urea
10. N-[3-(4-bromo-1-methyl-1h-pyrazol-5-yl)-4-methoxyphenyl]-n'-(2,4-difluorophenyl)-urea
11. Nelotanserin [usan:inn]
12. Unii-4za73qew2p
13. Apd125(nelotanserin)
14. Nelotanserin (usan/inn)
15. Nelotanserin [inn]
16. Nelotanserin [usan]
17. Schembl2345325
18. Apd 125
19. Dtxsid40232868
20. Chebi:177438
21. Bcp24992
22. Ex-a3147
23. Bdbm50324541
24. Mfcd16619341
25. Zinc38239930
26. Cs-5984
27. Db12555
28. Sb19023
29. As-30117
30. Hy-10559
31. D09645
32. 713b369
33. Q6990276
34. 1-[3-(4-bromo-2-methyl-2h-pyrazol-3-yl)-4-methoxy-phenyl]-3-(2,4-difluoro-phenyl)-urea
35. 1-[3-(4-bromo-2-methyl-2h-pyrazol-3-yl)-4-methoxy-phenyl]-3-(2,4-difluoro-phenyl)urea
36. 1-[3-(4-bromo-2-methylpyrazol-3-yl)-4-methoxyphenyl]-3-(2,4-diluorophenyl)urea
37. 1-(3-(4-bromo-2-methyl-2h-pyrazol-3-yl)-4-methoxyphenyl)-3-(2,4- Difluorophenyl)urea
38. Urea, N-(3-(4-bromo-1-methyl-1h-pyrazol-5-yl)-4-methoxyphenyl)-n'-(2,4-difluorophenyl)-
| Molecular Weight | 437.2 g/mol |
|---|---|
| Molecular Formula | C18H15BrF2N4O2 |
| XLogP3 | 3.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 4 |
| Exact Mass | 436.03464 g/mol |
| Monoisotopic Mass | 436.03464 g/mol |
| Topological Polar Surface Area | 68.2 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 518 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Nelotanserin potently and selectively targets the 5-HT2A serotonin receptor, blocking a stimulatory pathway of the central nervous system. This mechanism is not expected to have the side effects of the GABA-A treatments.
