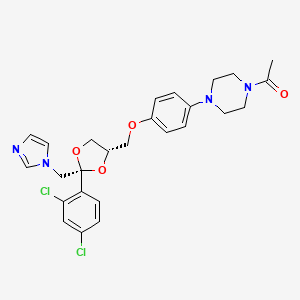

1. Ketoconazole
2. Nizoral
3. R 41400
4. R-41400
5. R41,400
6. R41400
1. Ketoconazole
2. (+)-ketoconazole
3. 65277-42-1
4. (2r,4s)-ketoconazole
5. 142128-59-4
6. Kuric
7. Ketocanazole
8. Cpd000058460
9. Chembl75
10. Smr000058460
11. Mls000069784
12. Mls001146934
13. 1-[4-[4-[[(2r,4s)-2-(2,4-dichlorophenyl)-2-(imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]piperazin-1-yl]ethanone
14. 1-acetyl-4-(4-{[(2r,4s)-2-(2,4-dichlorophenyl)-2-(1h-imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy}phenyl)piperazine
15. Chebi:48336
16. Dsstox_cid_9879
17. R-41400
18. Dsstox_rid_78829
19. Dsstox_gsid_29879
20. 1-(4-(4-(((2r,4s)-2-((1h-imidazol-1-yl)methyl)-2-(2,4-dichlorophenyl)-1,3-dioxolan-4-yl)methoxy)phenyl)piperazin-1-yl)ethanone
21. Mfcd00058579
22. Rac-trans-ketoconazole
23. Ktz
24. Sr-01000075626
25. Sr-01000597381
26. Ketoconazole (k)
27. (+)-ketoconazol
28. Ketoconazole,(s)
29. Ncgc00016907-01
30. Prestwick_744
31. Cas-65277-42-1
32. Tocris-1103
33. Opera_id_397
34. Prestwick0_000389
35. Prestwick1_000389
36. Prestwick2_000389
37. Prestwick3_000389
38. R 41,400
39. Upcmld-dp138
40. Schembl8407
41. Lopac0_000666
42. Bspbio_000577
43. Mls000758224
44. Mls001423987
45. Mls002207053
46. Mls002222255
47. Bidd:gt0696
48. Us9150527, Ketoconazole
49. Spbio_002498
50. Amy917
51. Bdbm8610
52. Bpbio1_000635
53. Dtxsid7029879
54. Upcmld-dp138:001
55. Bdbm60666
56. Hy-b0105a
57. Ketoconazole, >=98% (hplc)
58. Dtxsid901316748
59. Hms1569m19
60. Hms2051a19
61. Hms2089n05
62. Hms2096m19
63. Hms2234h17
64. Hms3262e13
65. Hms3414j19
66. Hms3678j17
67. Hms3713m19
68. Zinc643138
69. Bcp28528
70. Piperazine, (+/-)-1-acetyl-4-[4-[[(2r,4s)-2-(2,4-dichlorophenyl)-2-(1h-imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-, Rel-
71. Ketoconazole 2.0 Mg/ml In Methanol
72. Tox21_110676
73. Tox21_300267
74. Tox21_500666
75. Ei-107
76. S1353
77. Akos007930650
78. Ccg-100815
79. Cs-1846
80. Ks-1205
81. Lp00666
82. Nc00065
83. Sdccgsbi-0050645.p002
84. Ketoconazole 100 Microg/ml In Methanol
85. Mrf-0000100
86. 24f2-1,25(oh)d3
87. Ncgc00025000-01
88. Ncgc00025000-02
89. Ncgc00025000-03
90. Ncgc00025000-04
91. Ncgc00025000-05
92. Ncgc00025000-06
93. Ncgc00025000-07
94. Ncgc00025000-08
95. Ncgc00025000-09
96. Ncgc00025000-10
97. Ncgc00025000-14
98. Ncgc00025000-16
99. Ncgc00025000-28
100. Ncgc00253967-01
101. Ncgc00261351-01
102. ( Inverted Exclamation Marka)-ketoconazol
103. (+/-)-cis-1-acetyl-4-[4-[[2-(2,4-dichlorophenyl)-2-(imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]piperazine
104. Ac-15957
105. Cis-1-acetyl-4-[4-[[2-(2,4-
106. 3-dioxolan-4-yl)-methoxy]phenyl)piperazine
107. Ketoconazole 100 Microg/ml In Acetonitrile
108. Eu-0100666
109. K0045
110. Sw196888-4
111. Bim-0050645.0001
112. K 1003
113. M02048
114. 277k421
115. Ketoconazole, Antibiotic For Culture Media Use Only
116. Q-201267
117. Sr-01000075626-1
118. Sr-01000075626-4
119. Sr-01000597381-1
120. Sr-01000597381-6
121. Brd-k29113274-001-03-6
122. Brd-k29113274-001-11-9
123. Brd-k29113274-001-21-8
124. Q27121163
125. (+)-r 41400
126. Ketoconazole, British Pharmacopoeia (bp) Reference Standard
127. Ketoconazole, European Pharmacopoeia (ep) Reference Standard
128. Ketoconazole, United States Pharmacopeia (usp) Reference Standard
129. Dichlorophenyl)-2-(1h-imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-piperazine
130. Ketoconazole, Pharmaceutical Secondary Standard; Certified Reference Material
131. (2r,4s)-1-acetyl-4-(4-{[2-(2,4-dichlorophenyl)-2-(1h-imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy}phenyl)piperazine
132. 1-(4-(4-(((2r,4s)-2-((1h-imidazol-1-yl)methyl)-2-(2,4-dichlorophenyl)-1,3-dioxolan-4-yl)methoxy)phenyl)piperazin-1-yl)ethan-1-one
133. Cis-1-acetyl-4-[4-[[2-(2,4-dichlorophenyl)-2-(1h-imidazol-1-ylmethyl)-1,3-dioxolan-4-yl] Methoxy]phenyl]piperazine
134. Kz
135. Piperazine, (+)-1-acetyl-4-[4-[[2-(2,4-dichlorophenyl)-2-(1h-imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-
| Molecular Weight | 531.4 g/mol |
|---|---|
| Molecular Formula | C26H28Cl2N4O4 |
| XLogP3 | 4.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 7 |
| Exact Mass | 530.1487608 g/mol |
| Monoisotopic Mass | 530.1487608 g/mol |
| Topological Polar Surface Area | 69.1 Ų |
| Heavy Atom Count | 36 |
| Formal Charge | 0 |
| Complexity | 735 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 10 | |
|---|---|
| Drug Name | Extina |
| PubMed Health | Ketoconazole |
| Drug Label | EXTINA Foam contains 2% ketoconazole USP, an antifungal agent, in a thermolabile hydroethanolic foam for topical application.The chemical name for ketoconazole is piperazine, 1-acetyl-4-[4-[[2-(2,4-dichlorophenyl) -2-(1H-imidazol-l-ylmethyl)-1,3-di... |
| Active Ingredient | Ketoconazole |
| Dosage Form | Aerosol, foam |
| Route | Topical |
| Strength | 2% |
| Market Status | Prescription |
| Company | Delcor Abet |
| 2 of 10 | |
|---|---|
| Drug Name | Ketoconazole |
| PubMed Health | Ketoconazole |
| Drug Label | NIZORAL is a synthetic broad-spectrum antifungal agent available in scored white tablets, each containing 200 mg ketoconazole base for oral administration. Inactive ingredients are colloidal silicon dioxide, corn starch, lactose, magnesium stearate... |
| Active Ingredient | Ketoconazole |
| Dosage Form | Shampoo; Tablet; Cream; Aerosol, foam |
| Route | Oral; Topical |
| Strength | 200mg; 2% |
| Market Status | Prescription |
| Company | Teva; Taro; Fougera Pharms; Perrigo New York; Tolmar; Mylan; Perrigo Israel |
| 3 of 10 | |
|---|---|
| Drug Name | Nizoral |
| PubMed Health | Ketoconazole |
| Drug Label | NIZORAL is a synthetic broad-spectrum antifungal agent available in scored white tablets, each containing 200 mg ketoconazole base for oral administration. Inactive ingredients are colloidal silicon dioxide, corn starch, lactose, magnesium stearate... |
| Active Ingredient | Ketoconazole |
| Dosage Form | Shampoo |
| Route | Topical |
| Strength | 2% |
| Market Status | Prescription |
| Company | Janben Pharms |
| 4 of 10 | |
|---|---|
| Drug Name | Nizoral a-d |
| PubMed Health | Ketoconazole (On the skin) |
| Active Ingredient | Ketoconazole |
| Dosage Form | Shampoo |
| Route | Topical |
| Strength | 1% |
| Market Status | Over the Counter |
| Company | Mcneil Cons |
| 5 of 10 | |
|---|---|
| Drug Name | Xolegel |
| PubMed Health | Ketoconazole |
| Drug Label | XOLEGEL contains the antifungal agent ketoconazole USP at 2% in a topical anhydrous gel vehicle for topical administration.Chemically, ketoconazole is ()-cis-1-Acetyl-4-[p-[[2-(2,4-dichlorophenyl)-2-(1H-imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methox... |
| Active Ingredient | Ketoconazole |
| Dosage Form | Gel |
| Route | Topical |
| Strength | 2% |
| Market Status | Prescription |
| Company | Aqua Pharms |
| 6 of 10 | |
|---|---|
| Drug Name | Extina |
| PubMed Health | Ketoconazole |
| Drug Label | EXTINA Foam contains 2% ketoconazole USP, an antifungal agent, in a thermolabile hydroethanolic foam for topical application.The chemical name for ketoconazole is piperazine, 1-acetyl-4-[4-[[2-(2,4-dichlorophenyl) -2-(1H-imidazol-l-ylmethyl)-1,3-di... |
| Active Ingredient | Ketoconazole |
| Dosage Form | Aerosol, foam |
| Route | Topical |
| Strength | 2% |
| Market Status | Prescription |
| Company | Delcor Abet |
| 7 of 10 | |
|---|---|
| Drug Name | Ketoconazole |
| PubMed Health | Ketoconazole |
| Drug Label | NIZORAL is a synthetic broad-spectrum antifungal agent available in scored white tablets, each containing 200 mg ketoconazole base for oral administration. Inactive ingredients are colloidal silicon dioxide, corn starch, lactose, magnesium stearate... |
| Active Ingredient | Ketoconazole |
| Dosage Form | Shampoo; Tablet; Cream; Aerosol, foam |
| Route | Oral; Topical |
| Strength | 200mg; 2% |
| Market Status | Prescription |
| Company | Teva; Taro; Fougera Pharms; Perrigo New York; Tolmar; Mylan; Perrigo Israel |
| 8 of 10 | |
|---|---|
| Drug Name | Nizoral |
| PubMed Health | Ketoconazole |
| Drug Label | NIZORAL is a synthetic broad-spectrum antifungal agent available in scored white tablets, each containing 200 mg ketoconazole base for oral administration. Inactive ingredients are colloidal silicon dioxide, corn starch, lactose, magnesium stearate... |
| Active Ingredient | Ketoconazole |
| Dosage Form | Shampoo |
| Route | Topical |
| Strength | 2% |
| Market Status | Prescription |
| Company | Janben Pharms |
| 9 of 10 | |
|---|---|
| Drug Name | Nizoral a-d |
| PubMed Health | Ketoconazole (On the skin) |
| Active Ingredient | Ketoconazole |
| Dosage Form | Shampoo |
| Route | Topical |
| Strength | 1% |
| Market Status | Over the Counter |
| Company | Mcneil Cons |
| 10 of 10 | |
|---|---|
| Drug Name | Xolegel |
| PubMed Health | Ketoconazole |
| Drug Label | XOLEGEL contains the antifungal agent ketoconazole USP at 2% in a topical anhydrous gel vehicle for topical administration.Chemically, ketoconazole is ()-cis-1-Acetyl-4-[p-[[2-(2,4-dichlorophenyl)-2-(1H-imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methox... |
| Active Ingredient | Ketoconazole |
| Dosage Form | Gel |
| Route | Topical |
| Strength | 2% |
| Market Status | Prescription |
| Company | Aqua Pharms |
Antifungal agents
National Library of Medicine's Medical Subject Headings. Ketoconazole. Online file (MeSH, 2014). Available from, as of August 28, 2014: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
Nizoral Tablets should be used only when other effective antifungal therapy is not available or tolerated and the potential benefits are considered to outweigh the potential risks. Nizoral (ketoconazole) Tablets are indicated for the treatment of the following systemic fungal infections in patients who have failed or who are intolerant to other therapies: blastomycosis, coccidioidomycosis, histoplasmosis, chromomycosis, and paracoccidioidomycosis. Nizoral Tablets should not be used for fungal meningitis because it penetrates poorly into the cerebrospinal fluid. /Included in US product label/
NIH; DailyMed. Current Medication Information for Nizoral (Ketoconazole) Tablet (Revised: March 2014). Available from, as of November 11, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=090660c1-6e6d-457f-adb5-046ddfcd1465
Oral ketoconazole has been used for the palliative treatment of Cushing's syndrome (hypercortisolism), including adrenocortical hyperfunction associated with adrenal or pituitary adenoma or ectopic corticotropin-secreting tumors. Based on ketoconazole's endocrine effects, the drug has been used in the treatment of advanced prostatic carcinoma. Safety and efficacy of ketoconazole have not been established for either of these indications. Oral ketoconazole also has been used in the treatment of hypercalcemia in patients with sarcoidosis and the treatment of tuberculosis-associated hypercalcemia and idiopathic infantile hypercalcemia and hypercalciuria. /NOT included in US product label/
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014
Ketoconazole has been used for the treatment of sporotrichosis caused by Sporothrix schenckii; however, the drug is not recommended since it is less effective and associated with more adverse effects than some other azoles. Oral itraconazole is considered the drug of choice for the treatment of cutaneous, lymphocutaneous, or mild pulmonary or osteoarticular sporotrichosis and for follow-up therapy in more severe infections after a response has been obtained with IV amphotericin B. /NOT included in US product label/
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014
For more Therapeutic Uses (Complete) data for KETOCONAZOLE (18 total), please visit the HSDB record page.
/BOXED WARNING/ WARNING. Nizoral Tablets should be used only when other effective antifungal therapy is not available or tolerated and the potential benefits are considered to outweigh the potential risks. Hepatotoxicity: Serious hepatotoxicity, including cases with a fatal outcome or requiring liver transplantation has occurred with the use of oral ketoconazole. Some patients had no obvious risk factors for liver disease. Patients receiving this drug should be informed by the physician of the risk and should be closely monitored. QT Prolongation and Drug Interactions Leading to QT Prolongation: Co-administration of the following drugs with ketoconazole is contraindicated: dofetilide, quinidine, pimozide, cisapride, methadone, disopyramide, dronedarone, ranolazine. Ketoconazole can cause elevated plasma concentrations of these drugs and may prolong QT intervals, sometimes resulting in life-threatening ventricular dysrhythmias such as torsades de pointes.
NIH; DailyMed. Current Medication Information for Nizoral (Ketoconazole) Tablet (Revised: March 2014). Available from, as of November 11, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=090660c1-6e6d-457f-adb5-046ddfcd1465
Transient increases in serum AST, ALT, and alkaline phosphatase concentrations may occur during ketoconazole therapy. Serious hepatotoxicity has occurred in patients receiving oral ketoconazole, including cases that were fatal or required liver transplantation. Hepatotoxicity may be hepatocellular (in most cases), cholestatic, or a mixed pattern of injury. Although ketoconazole-induced hepatotoxicity usually is reversible following discontinuance of the drug, recovery may take several months and rarely death has occurred. Symptomatic hepatotoxicity usually is apparent within the first few months of ketoconazole therapy, but occasionally may be apparent within the first week of therapy. Some patients with ketoconazole-induced hepatotoxicity had no obvious risk factors for liver disease. Serious hepatotoxicity has been reported in patients receiving high oral ketoconazole dosage for short treatment durations and in patients receiving low oral dosage of the drug for long durations. Many of the reported cases of hepatotoxicity occurred in patients who received the drug for the treatment of tinea unguium (onychomycosi or the treatment of chronic, refractory dermatophytoses. Ketoconazole-induced hepatitis has been reported in some children.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 521
Coadministration of a number of CYP3A4 substrates such as dofetilide, quinidine cisapride and pimozide is contraindicated with Nizoral Tablets. Coadministration with ketoconazole can cause elevated plasma concentrations of these drugs and may increase or prolong both therapeutic and adverse effects to such an extent that a potentially serious adverse reaction may occur. For example, increased plasma concentrations of some of these drugs can lead to QT prolongation and sometimes resulting in life-threatening ventricular tachyarrhythmias including occurrences of torsades de pointes, a potentially fatal arrhythmia. Additionally, the following other drugs are contraindicated with Nizoral Tablets: methadone, disopyramide, dronedarone, ergot alkaloids such as dihydroergotamine, ergometrine, ergotamine, methylergometrine, irinotecan, lurasidone, oral midazolam, alprazolam, triazolam, felodipine, nisoldipine, ranolazine, tolvaptan, eplerenone, lovastatin, simvastatin and colchicine.
NIH; DailyMed. Current Medication Information for Nizoral (Ketoconazole) Tablet (Revised: March 2014). Available from, as of November 11, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=090660c1-6e6d-457f-adb5-046ddfcd1465
The use of Nizoral Tablets is contraindicated in patients with acute or chronic liver disease.
NIH; DailyMed. Current Medication Information for Nizoral (Ketoconazole) Tablet (Revised: March 2014). Available from, as of November 11, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=090660c1-6e6d-457f-adb5-046ddfcd1465
For more Drug Warnings (Complete) data for KETOCONAZOLE (46 total), please visit the HSDB record page.
Ketoconazole HRA is indicated for the treatment of endogenous Cushings syndrome in adults and adolescents above the age of 12 years.
Cytochrome P-450 CYP3A Inhibitors
Drugs and compounds which inhibit or antagonize the biosynthesis or actions of CYTOCHROME P-450 CYP3A. (See all compounds classified as Cytochrome P-450 CYP3A Inhibitors.)
14-alpha Demethylase Inhibitors
Compounds that specifically inhibit STEROL 14-DEMETHYLASE. A variety of azole-derived ANTIFUNGAL AGENTS act through this mechanism. (See all compounds classified as 14-alpha Demethylase Inhibitors.)
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues. (See all compounds classified as Antifungal Agents.)
J02AB02
D01AC08
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
D - Dermatologicals
D01 - Antifungals for dermatological use
D01A - Antifungals for topical use
D01AC - Imidazole and triazole derivatives
D01AC08 - Ketoconazole
G - Genito urinary system and sex hormones
G01 - Gynecological antiinfectives and antiseptics
G01A - Antiinfectives and antiseptics, excl. combinations with corticosteroids
G01AF - Imidazole derivatives
G01AF11 - Ketoconazole
H - Systemic hormonal preparations, excl. sex hormones and insulins
H02 - Corticosteroids for systemic use
H02C - Antiadrenal preparations
H02CA - Anticorticosteroids
H02CA03 - Ketoconazole
J - Antiinfectives for systemic use
J02 - Antimycotics for systemic use
J02A - Antimycotics for systemic use
J02AB - Imidazole derivatives
J02AB02 - Ketoconazole
Ketoconazole is rapidly absorbed from the GI tract. Following oral administration, ketoconazole is dissolved in gastric secretions and converted to the hydrochloride salt prior to absorption from the stomach.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014
The effect of food on the rate and extent of GI absorption of ketoconazole has not been clearly determined. Some clinicians have reported that administration of ketoconazole to fasting individuals results in higher plasma concentrations of the drug than does administration with food. However, the manufacturer states that administration of ketoconazole with food increases the extent of absorption and results in more consistent plasma concentrations of the drug. The manufacturer suggests that food increases absorption of ketoconazole by increasing the rate and/or extent of dissolution of ketoconazole (e.g., by increasing bile secretions) or by delaying stomach emptying.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014
Ketoconazole is a weak dibasic agent and thus requires acidity for dissolution and absorption.
NIH; DailyMed. Current Medication Information for Nizoral (Ketoconazole) Tablet (Revised: March 2014). Available from, as of November 11, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=090660c1-6e6d-457f-adb5-046ddfcd1465
The bioavailability of oral ketoconazole depends on the pH of the gastric contents in the stomach; an increase in the pH results in decreased absorption of the drug. Decreased bioavailability of ketoconazole has been reported in patients with acquired immunodeficiency syndrome (AIDS), probably because of gastric hypochlorhydria associated with this condition; concomitant administration of dilute hydrochloric acid solution normalized absorption of the drug in these patients.198 Concomitant administration of an acidic beverage may increase bioavailability of oral ketoconazole in some individuals with achlorhydria.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014
For more Absorption, Distribution and Excretion (Complete) data for KETOCONAZOLE (19 total), please visit the HSDB record page.
Ketoconazole is partially metabolized, in the liver, to several inactive metabolites by oxidation and degradation of the imidazole and piperazine rings, by oxidative O-dealkylation, and by aromatic hydroxylation.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014
Plasma concentrations of ketoconazole appear to decline in a biphasic manner with a half-life of approximately 2 hours in the initial phase and approximately 8 hours in the terminal phase.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014
Elimination from plasma is biphasic with a half-life of 2 hours during the first 10 hours and 8 hours thereafter.
NIH; DailyMed. Current Medication Information for Nizoral (Ketoconazole) Tablet (Revised: March 2014). Available from, as of November 11, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=090660c1-6e6d-457f-adb5-046ddfcd1465
Ketoconazole blocks the synthesis of ergosterol, a key component of the fungal cell membrane, through the inhibition of cytochrome P-450 dependent enzyme lanosterol 14alpha-demethylase responsible for the conversion of lanosterol to ergosterol in the fungal cell membrane. This results in an accumulation of methylated sterol precursors and a depletion of ergosterol within the cell membrane thus weakening the structure and function of the fungal cell membrane.
NIH; DailyMed. Current Medication Information for Nizoral (Ketoconazole) Tablet (Revised: March 2014). Available from, as of November 11, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=090660c1-6e6d-457f-adb5-046ddfcd1465
Like other azole antifungal agents, ketoconazole presumably exerts its antifungal activity by altering cellular membranes, resulting in increased membrane permeability, secondary metabolic effects, and growth inhibition. Although the exact mechanism of action of ketoconazole has not been fully determined, it has been suggested that the fungistatic activity of the drug may result from interference with ergosterol synthesis, probably via inhibition of C-14 demethylation of sterol intermediates (e.g., lanosterol). The fungicidal activity of ketoconazole at high concentrations may result from a direct physiochemical effect of the drug on the fungal cell membrane.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014