

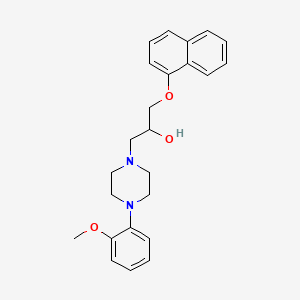

1. 1-(4-(2-methoxyphenyl)-1-piperazinyl)-3-(1-naphthyloxy)-2-propanol
1. 57149-07-2
2. Flivas
3. Kt-611
4. Naftopidil [inn]
5. Avishot
6. 1-(4-(2-methoxyphenyl)piperazin-1-yl)-3-(naphthalen-1-yloxy)propan-2-ol
7. Bm-15275
8. Naftopidil (flivas)
9. R9phw59sfn
10. Nsc-759293
11. Chembl142635
12. Ncgc00015718-06
13. 1-[4-(2-methoxyphenyl)piperazin-1-yl]-3-(naphthalen-1-yloxy)propan-2-ol
14. Dsstox_cid_25176
15. Dsstox_rid_80725
16. Dsstox_gsid_45176
17. Naftopidilum [latin]
18. Naftopidilum
19. 4-(2-methoxyphenyl)-alpha-[(1-naphthalenyloxy)methyl]-1-piperazineethanol
20. Naftopidil Hydrochloride Hydrate
21. Smr000466346
22. Cas-57149-07-2
23. Naftopidil (unspecified)
24. Unii-r9phw59sfn
25. Brn 0629965
26. Naftopidil,(s)
27. Flivas (tn)
28. Naftopidil [mi]
29. Naftopidil [jan]
30. Prestwick0_000975
31. Prestwick1_000975
32. Prestwick2_000975
33. Prestwick3_000975
34. (+-)-1-(4-(2-methoxyphenyl)piperazinyl)-3-(1-naphthyloxy)propan-2-ol
35. Naftopidil (jp17/inn)
36. (rs)-1-(4-(2-methoxyphenyl)-1-piperazinyl)-3-(1-naphthyloxy)-2-propanol
37. 4-(2-methoxyphenyl)-alpha-((1-naphthalenyloxy)methyl)-1-pioerazineethanol
38. Cid_4418
39. Naftopidil [mart.]
40. Naftopidil [who-dd]
41. Lopac0_000941
42. Regid_for_cid_4418
43. Bspbio_001009
44. Mls000759459
45. Mls001424117
46. Schembl113215
47. Spbio_002920
48. Bpbio1_001111
49. Dtxsid5045176
50. Bdbm50773
51. Chebi:31891
52. Cid_6603044
53. Hms2051b09
54. Hms2089g07
55. Hms3393b09
56. Hms3655h21
57. Hms3884p07
58. Pharmakon1600-01506024
59. Bcp21786
60. Hy-b0391
61. 3-(naphthalen-1-yloxy)propan-2-ol
62. Tox21_110205
63. Bbl028454
64. Mfcd00242741
65. Nsc759293
66. S2126
67. Stl372645
68. (+-)-4-(o-methoxyphenyl)-alpha-((1-naphthyloxy)methyl)-1-piperazineethanol
69. 1-[4-(2-methoxyphenyl)piperazin-1-yl]-3-naphthalen-1-yloxypropan-2-ol
70. 1-pioerazineethanol, 4-(2-methoxyphenyl)-alpha-((1-naphthalenyloxy)methyl)-
71. Akos002279617
72. Akos016328058
73. Tox21_110205_1
74. Bcp9000976
75. Ccg-100935
76. Ccg-205022
77. Db12092
78. Fe-0209
79. Nc00185
80. Nsc 759293
81. Sb19321
82. Sdccgsbi-0050915.p003
83. Ncgc00015718-04
84. Ncgc00015718-05
85. Ncgc00015718-08
86. Ncgc00015718-09
87. Ncgc00015718-10
88. Ncgc00015718-12
89. Ncgc00015718-25
90. Ncgc00024672-03
91. Ncgc00024672-04
92. Bn164631
93. 1-(4-(2-methoxyphenyl)piperazin-1-yl)-
94. Sbi-0050915.p002
95. Ab00514643
96. Ft-0630696
97. N0832
98. Sw196570-4
99. 49n072
100. D01674
101. Ab00489961-18
102. Ab00489961-19
103. Ab00489961_20
104. Ab00489961_21
105. A831328
106. Q6958243
107. Brd-a01787639-300-03-7
108. Brd-a01787639-300-04-5
109. 1-[4-(2-methoxyphenyl)piperazinyl]-3-(1-naphthyloxy)propan-2-ol
110. 1-[4-(2-methoxyphenyl)-1-piperazinyl]-3-(1-naphthalenyloxy)-2-propanol
111. 1-[4-(2-methoxyphenyl)piperazino]-3-(1-naphthoxy)propan-2-ol;hydrochloride
112. Bm-15275; Kt-611; Bm 15275; Kt 611; Bm15275; Kt611
113. (+/-)-4-(o-methoxyphenyl)-.alpha.-((1-naphthyloxy)methyl)-1-piperazineethanol
114. 1-[4-(2-methoxyphenyl)-1-piperazinyl]-3-(1-naphthalenyloxy)-2-propanol;hydrochloride
115. 1-[4-(2-methoxyphenyl)piperazin-1-yl]-3-naphthalen-1-yloxy-propan-2-ol;hydrochloride
116. 1-[4-(2-methoxyphenyl)piperazin-1-yl]-3-naphthalen-1-yloxypropan-2-ol;hydrochloride
| Molecular Weight | 392.5 g/mol |
|---|---|
| Molecular Formula | C24H28N2O3 |
| XLogP3 | 4.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 7 |
| Exact Mass | 392.20999276 g/mol |
| Monoisotopic Mass | 392.20999276 g/mol |
| Topological Polar Surface Area | 45.2 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 483 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Adrenergic alpha-Antagonists
Drugs that bind to but do not activate alpha-adrenergic receptors thereby blocking the actions of endogenous or exogenous adrenergic agonists. Adrenergic alpha-antagonists are used in the treatment of hypertension, vasospasm, peripheral vascular disease, shock, and pheochromocytoma. (See all compounds classified as Adrenergic alpha-Antagonists.)
Calcium Channel Blockers
A class of drugs that act by selective inhibition of calcium influx through cellular membranes. (See all compounds classified as Calcium Channel Blockers.)
Platelet Aggregation Inhibitors
Drugs or agents which antagonize or impair any mechanism leading to blood platelet aggregation, whether during the phases of activation and shape change or following the dense-granule release reaction and stimulation of the prostaglandin-thromboxane system. (See all compounds classified as Platelet Aggregation Inhibitors.)
